Abstract
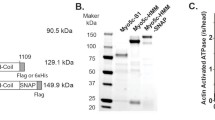
Long myosin light chain kinase (L-MLCK) contains five DFRXXL motifs with ability to bind F-actin. Binding stoichiometry data indicated that each DFRXXL motif might bind each G-actin, but its biological significance remained unknown. We hypothesized that L-MLCK might act as an F-actin bundle peptides by its multiple binding sites of 5DFRXXL motifs to actin. In order to characterize F-actin-bundle formation properties of 5DFRXXL region of long myosin light chain kinase, we expressed and purified 5DFRXXL peptides tagged with HA in vitro. The properties of 5DFRXXL peptides binding to myofilaments or F-actin were analyzed by binding stoichiometries assays. The results indicated that 5DFRXXL peptides bound to myofilaments or F-actin with high affinity. KD values of 5DFRXXL binding to myofilaments and F-actin were 0.45 and 0.41 μmol/L, respectively. Cross-linking assay demonstrated that 5DFRXXL peptides could bundle F-actin efficiently. Typical F-actin bundles were observed morphologically through determination of confocal and electron microscopy after adding 5DFRXXL peptides. After transfection of pEGFP-5DFRXXL plasmid into eukaryocyte, spike structure was observed around cell membrane edge. We guess that such structure formation may be attributable to F-actin over-bundle formation caused by 5DFRXXL peptides. Therefore, we suppose that L-MLCK may be a new bundling protein and somehow play a certain role in organization of cell skeleton besides mediating cell contraction by it kinase activity.
Similar content being viewed by others
References
Kamm, K. E., Stull, J. T., The function of myosin and myosin light chain kinase phosphorylation in smooth muscle, Annu. Rev. Pharmacol. Toxicol, 1985, 25: 593–620.
Hartshorne, D. J., Biochemistry of the contractile process in smooth muscle, in Physiology of the Gastrointestinal Tract (ed. Johnson, L. R.), New York: Raven Press, 1987, 423–482.
Stull, J. T., Lin, P. J., Krueger, J. K. et al., Myosin light chain kinase: Functional domains and structural motifs, Acta Physiol. Scand, 1998, 164(n4): 471–482.
Kamm, K. E., Stull, J. T., Dedicated myosin light chain kinases with diverse cellular functions, J. Biol. Chem., 2001, 276(n7): 4527–30.
Schoenwaelder, S. M., Burridge, K., Bidirectional signaling between the cytoskeleton and integrins, Curr. Opin. Cell Biol, 1999, 11(n2): 274–286.
Bresnick, A. R., Molecular mechanisms of nonmuscle myosin-II regulation, Curr. Opin. Cell Biol, 1999, 11(n1): 26–33.
Sato, M., Tani, E., Fujikawa, H. et al., Involvement of Rhokinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm, Circ. Res., 2000, 87: 195–200.
Van Nieuw-Amerongen, G. P., van Delft, S., Vermeer, M. A. et al., Activation of RhoA by thrombin in endothelial hypermeability: Role of Rho kinase and protein tyrosine kinase, Circ. Res., 2000, 87(n4): 335–340.
Jung, C., Chylinski, T. M., Pimenta, A. et al., Neurofilament transport is dependent on actin and myosin, J. Neurosci., 2004, 24(n43): 9486–9496.
Clayburgh, D. R., Rosen, S., Witkowski, E. D. et al., A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability, J. Biol. Chem., 2004, 279(n53): 55506–55513.
Tran, Q. K., Watanabe, H., Zhang, X. X. et al., Involvement of myosin light-chain kinase in chloride-sensitive Ca2+ influx in porcine aortic endothelial cells, Cardiovasc. Res., 1999, 44(n3): 623–631.
Szaszi, K., Kurashima, K., Kapus, A. et al., RhoA and Rho kinase regulate the epithelial Na+/H+ exchanger NHE3, Role of myosin light chain phosphorylation, J. Biol. Chem., 2000, 275(n37): 28599–28606.
Aromolaran, A. S., Albert, A. P., Large, W. A., Evidence for myosin light chain kinase mediating noradrenaline-evoked cation current in rabbit portal vein myocytes, J. Physiol. (Lond.), 2000, 524(n3): 853–863.
Ammit, A. J., Armour, C. L., Black, J. L., Myosin light chain kinase content is increased in human sensitised airway smooth muscle, Am. J. Respir. Crit. Care Med., 2000, 161(n1): 257–263.
Smith, L., Su, X., Lin, P. et al., Identification of a novel actin binding motif smooth muscle myosin light chain kinase, J. Biol. Chem., 1999, 274(n41): 29433–29438.
Smith, L., Stull, J. T., Myosin light chain kinase binding to actin filaments, FEBS Lett., 2000, 480(n2–3): 298–300.
Smith, L., Parizi-Robinson, M., Zhu, M. S. et al., Properties of long myosin light chain kinase binding to F-actin in vitro and in vivo, J. Biol. Chem., 2002, 277(n38): 35597–35604.
Lin, P. J., Luby-Phelps, K., Stull, J. T., Binding of myosin light chain kinase to cellular actin-myosin filaments, J. Biol. Chem., 1997, 272(n11): 7412–7420.
Wang, Z., Meng, X. M., Cao, H. Q. et al., Characteristics of the binding features of Nelin with F-actin and screening Nelin interactive proteins, Chin. Sci. Bull., 2004, 49(n23): 2487–2490.
Pollard, T. D., Goldberg, I., Schwarz, W. H., Nucleotide exchange, structure, and mechanical properties of filaments assembled from ATP-actin and ADP-actin, J. Biol. Chem., 1999, 267(n28): 20339–20345.
Shin, J. H., Gardel, M. L., Mahadevan, L. et al., Relating microstructure to rheology of a bundled and cross-linked F-actin network in vitro, Proc. Natl. Acad. Sci. USA, 2004, 101(n26): 9636–9641.
Kudryashov, D. S., Chibalina, M. V., Birukov, K. G. et al., Unique sequence of a high molecular weight myosin light chain kinase is involved in interaction with actin cytoskeleton, FEBS Lett., 1999, 463(n1–2): 67–71.
Poperechnaya, A., Varlamova, O., Lin, P. J. et al., Localization and activity of myosin light chain kinase isoforms during the cell cycle, J. Cell Biol., 2000, 151(n3): 697–708.
Small, J. V., Stradal, T., Vignal, E. et al., The lamellipodium: Where motility begins, Trends Cell Biol., 2002, 12(n3): 112–120.
Melinda, L., Michel, L. T., Kenneth, M. Y., Phosphatases in cell-matrix adhesion and migration, Nat. Rev. Mol. Cell Biol., 2003, 4: 700–711.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yang, C., Wei, D., Chen, C. et al. 5DFRXXL region of long myosin light chain kinase causes F-actin bundle formation. Chin.Sci.Bull. 50, 2045–2051 (2005). https://doi.org/10.1007/BF03322799
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03322799