Summary
Introduction
Compliance is crucial in the treatment with statins. It is generally evaluated by comparing the daily dose received on average by treated patients with a standard parameter, which is often the corresponding DDD (Defined Daily Dose) issued by WHO (World Health Organisation).
Materials and methods
As regards statins, the DDDs issued by WHO (which will be hereafter referred to as WHO-DDDs) have shown to be poor indicators of a correct dosing (which they generally underestimate). As an alternative evaluation standard, reference based DDDs were drawn, for this study, from NCEP (National Cholesterol Education Program) guidelines, i.e. from in progress clinical research on efficacy and safety of statins. This multicenter, retrospective study is based on a statistical sample including six ASLs (ASL: Azienda Sanitaria Locale, Local Health Unit), located in as many Italian Regions, on the whole providing health care for 2,625,000 people. Subjects were eligible to whom statins had been prescribed at least once in the period 2001–2005 (observation period).
Results
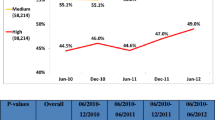
Data relative to 294,295 patients were selected from the ASL administrative databases. Sampled patients were aged 61 on average and 48% males. The patient’s compliance (measured as the ratio between the average daily dose and the reference based DDD) increased on the whole from less than 40% to more than 50% in the observation period. (Had the compliance been evaluated with WHO-DDDs, the analogous values would have been 85% and 100%). The compliance was analysed through appropriateness bands. In particular, the frequency of quite incorrectly-treated patients (compliance up to 25%) decreased from 65% to 50% of the whole sample, while the frequency of patients in the optimal treatment band (75% to 100% compliance) soared from 3% to 10%.
Discussion and conclusions
The ASLs were chosen based on availability and reliability criteria, which resulted in their being concentrated in the Northern-Central part of Italy (with the Marche Region weighing more than half of the sample). They could nevertheless be considered as fairly representative of the whole country. The compliance constantly improved during the observation period, even if it reached (only) 50% on the overall average in 2005. If the WHO-DDDs had been used, the compliance would have been too optimistically evaluated, even suggesting an over-treatment area with statins (resulting in the high frequency of patients included in the band with more than 100% compliance).
Similar content being viewed by others
Bibliografia
National Center for Chronic Disease Prevention and Health Promotion. Preventing heart disease and stroke (online: www.cdc.gov/nccdphp/bb_heartdisease/index.htm)
Task Force for Compliance. Non compliance with medication regimens: an economic tragedy: emerging issues in pharmaceutical cost containing. Washington, DC: National Pharmaceutical Council, 1994: 1–32
Mc Ghan WF, Peterson AM. Pharmacoeconomic impact of non-compliance. Bridgewater, NJ: US Pharmacist Impact, 2001: 3–13
Stamler J, Wentwoeth D, Neaton JD. Prevalence and prognostic significance of hypercholesterolemia in men with hypertension: prospective data on primary screens of the Multiple Risk Factor Intervention Trial. Am J Med 1986; 80: 33–9
Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279: 1615–22
Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia: West of Scotland Coronary Prevention Study Group. N Engl J Med 1995; 333: 1301–7
Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383–9
The Long-Term Intervention with pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339: 1349–57
Wei L, Wang J, Thompson P, et al. Adherence to statin treatment and readmission of patients after myocardial infarction: a six year follow up study. Heart 2002; 88: 229–33
Avorn J, Monett J, Lacour A, et al. Persistence of use lipid-lowering medications: a cross-national study. JAMA 1998; 279: 1458–62
Lucioni C, Mazzi S, Cerra C, et al. Uno studio di drug utilisation delle statine nella recente prassi terapeutica italiana. PharmacoEconomics-Italian Research Articles 2006; 8: 3–17
Lucioni C, Mazzi S, Cerra C, Fratino P. Gli Inibitori della Pompa Protonica: uno studio di drug utilization, con particolare riferimento all’appropriatezza del loro uso nella gastroprotezione. Pharmacoeconomics-Italian Research Articles 2007; 9: 75–89
Clark KW, Gray D. The Defined Daily Dose as a tool in Pharma coeconomics. Advantages and limitations. PharmacoEconomics 1995; 7: 280–3
Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systemic review and meta-analysis. BMJ 2003; 326: 1423–9
Grundy SM, Cleeman JI, Noel Bairy Merz C, et al., for the Coordinating Committee of the National Cholesterol Education Program. Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation 2004; 110: 227–39
Catapano A, Brady WE, King TR, Palmisano J. Lipid alteringefficacy of ezetimibe co-administered with simvastatin compared with rosuvastatin: a meta-analysis of pooled data from 14 clinical trials. Curr Med Res Opin 2005; 21: 1123–30
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360: 7–22
Deambrosis P, Saramin C, Terrazzani G, et al. Dieci anni di utilizzo delle statine: adesione alla terapia e costi del trattamento farmacologico. Pharmacoeconomics-Italian Research Articles 2005; 7: 187–94
Walley T, Folino-Gallo P, Stephens P, Van Ganse E. Trends in prescribing and utilization of statins and other lipid lowering drugs across Europe 1997–2003. Br J Clin Pharmacol 2005; 60: 543–51
Peterson AM, Nau PN, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007; 10: 3–12
Author information
Authors and Affiliations
Corresponding author
Additional information
Il Board Scientifico dello studio è composto da: O. Brignoli (Vice Presidenza SIMG), M. Fagotti (Servizio Farmaceutico, ASL Foligno), C. Lucioni (Wolters Kluwer Health), G. Rossi (Direzione Sanitaria, ASL Lecco), C. Schweiger (Cardiologo)
I peer reviewers, per questo articolo, sono stati coordinati da Patrizia Berto.
Rights and permissions
About this article
Cite this article
Lucioni, C., Mazzi, S., Pari, B. et al. L’utilizzo delle DDD reference based per la valutazione della compliance terapeutica nel trattamento con statine. Pharmacoeconomics-Ital-Res-Articles 10, 77–88 (2008). https://doi.org/10.1007/BF03320644
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03320644