Abstract
The Toward Integrated Treatment of Advanced Hepatocellular Carcinoma with Nexavar (TiTAN) Symposium was held in August 2010 in Tokyo, Japan, during which the position of sorafenib (Nexavar®) in the treatment of HCC in Japan (for which it received approval in 2009) was discussed by a panel of eight expert hepatologists in a session chaired by Dr Kudo. The following article focuses on the discussion that went on during this session, including question and answer sessions regarding the experiences of the 350 conference attendees in treating patients with HCC, as well as some of the more challenging disease management issues.
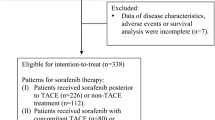
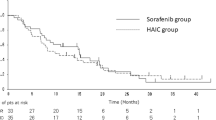
Since 2008, when the phase III Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial demonstrated an increase in the median overall survival (OS) for patients with unresectable HCC treated with sorafenib compared with placebo, international and Japanese guidelines recommend sorafenib as a first-line option for patients with advanced HCC Child-Pugh liver function class A who have extrahepatic metastasis. Sorafenib is also recommended for patients unresponsive to transarterial chemoembolization (TACE) or hepatic arterial infusion chemotherapy (HAIC). Importantly, if HCC is judged to be unresponsive to TACE, treatment should be switched to sorafenib in a timely manner.
Almost half of the conference attendees said that they used both the Japan Society of Hepatology clinical practice guidelines and the clinical practice guidelines for HCC when determining treatment strategies for individual HCC patients. Sorafenib should currently not be used as adjuvant therapy or in combination with TACE or HAIC until evidence from ongoing clinical trials shows that it is beneficial in these settings.
Similar content being viewed by others
References
Bayer Healthcare Pharmaceuticals. Nexavar approved in Japan for the treatment of advanced liver cancer. 2009 [cited; Available from: http://press.bayerhealthcare.com/en/press/auth/news-details-page.php/13182/2009-0239
Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatology International 2010; 4(2): 439–74
The Japan Society of Hepatology. The Japanese HCC Clinical Practice Guideline. Treatment algorithm for hepatocellular carcinoma. Hepatology Research 2010; 40 (Suppl. 1): 8–9
Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 2011; 29(3): 339–64
Bruix J, Sherman M. AASLD Practice Guideline: Management of Hepatocellular Carcinoma: An Update. Available at http://www.aasld.org/practiceguidelines/Documents/Bookmarked%20Practice%20Guidelines/HCCUpdate2010.pdf (Accessed 14 Nov 2010). Hepatology 2010; July: 1–35
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003; 362(9399): 1907–17
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359(4): 378–90
Sherman M, Mazzaferro V, Amadori D, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma and vascular invasion or extrahepatic spread: A subanalysis from the SHARP trial. J Clin Oncol 2008; 26 (May 20 suppl): abstract 4584
Kudo M, Ueshima K. Positioning of a molecular-targeted agent, sorafenib, in the treatment algorithm for hepatocellular carcinoma and implication of many complete remission cases in Japan. Oncology 2010 Jul; 78 (Suppl. 1): 154–66
Furuse J, Okusaka T, Kaneko S, et al. Phase I/II study of the pharmacokinetics, safety and efficacy of S-1 in patients with advanced hepatocellular carcinoma. Cancer Sci 2010; 101(12): 2606–11
Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008 Oct; 48(4): 1312–27
Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology 2000; 32(6): 1224–9
Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005; 129(1): 122–30
Charnsangavej C, Chuang VP, Wallace S, et al. Angiographic classification of hepatic arterial collaterals. Radiology 1982; 144(3): 485–94
Ikeda K, Kumada H, Saitoh S, et al. Effect of repeated trans-catheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer 1991; 68(10): 2150–4
Kawamura Y, Ikeda K, Hirakawa M, et al. Efficacy of platinum analogue for advanced hepatocellular carcinoma unresponsive to transcatheter arterial chemoembolization with epirubicin. Hepatol Res 2009; 39(4): 346–54
Maeda N, Osuga K, Higashihara H, et al. Transarterial chemoembolization with cisplatin as second-line treatment for hepatocellular carcinoma unresponsive to chemoembolization with epirubicin-lipiodol emulsion. Cardiovasc Intervent Radiol 2011 Feb; 35(1): E82–9
Galle P, Blanc J, Van Laethem J-L, et al. Efficacy and safety of sorafenib in patients with hepatocellular carcinoma and prior anti-tumor therapy: a subanalysis from the SHARP trial. 43rd annual meeting of the European Association for the Study of the Liver (EASL 2008) Milan, Italy April 23–27, 2008 2008 [cited; Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18817997
Iwasa S, Ikeda M, Okusaka T, et al. Transcatheter arterial infusion chemotherapy with a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. Jpn J Clin Oncol 2011 Jun; 41(6): 770–5
Bayer HealthCare Pharmaceuticals Inc. Sorafenib. Prescribing information. Available at http://www.nexavar.com/html/download/Nexavar_PI.pdf Accessed 2 December 2010. 2009
Ueshima K, Kudo M, Tanaka M, et al. Session 11-05, Phase I study of sorafenib in combination with low-dose cisplatin and fluorouracil intra-arterail infusion chemotherapy. Osaka, Japan: The 2nd Asia-Pacific Primary Liver Cancer Expert Meeting-A Bridge to Consensus on HCC Management, 2011
Kudo M. Molecular Targeted Therapy of Hepatocellular Carcinoma. Tokyo: Arc Media, 2010
Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011; (14): 2117–27
Vagefi PA, Hirose R. Downstaging of hepatocellular carcinoma prior to liver transplant: is there a role for adjuvant sorafenib in locoregional therapy? J Gastrointest Cancer 2010; 41(4): 217–20
Saab S, McTigue M, Finn RS, et al. Sorafenib as adjuvant therapy for high-risk hepatocellular carcinoma in liver transplant recipients: feasibility and efficacy. Exp Clin Transplant 2010; 8(4): 307–13
Yeganeh M, Finn RS, Saab S. Apparent remission of a solitary metastatic pulmonary lesion in a liver transplant recipient treated with sorafenib. Am J Transplant 2009; 9(12): 2851–4
Bhoori S, Toffanin S, Sposito C, et al. Personalized molecular targeted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol 2010; 52(5): 771–5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kudo, M., Tateishi, R., Yamashita, T. et al. Current Status of Hepatocellular Carcinoma Treatment in Japan. Clin Drug Investig 32 (Suppl 2), 37–51 (2012). https://doi.org/10.1007/BF03265495
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03265495