Abstract
Background
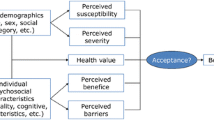
Understanding the link between patients’ beliefs and behavior may help explain their attitude to their treatment. How patients’ personal experience of their treatment results in their decision to accept taking it or not and to persist in taking it remains to be explored more thoroughly. Acceptance is hypothesized to be the balance patients establish between their medication’s advantages and its disadvantages, based on their personal experience with the medication. Measuring patients’ acceptance of their medication is likely to predict their behavior (adherence and persistence) towards their treatment.
Objective
Our objective was to develop a generic medication acceptance measure assessing how patients weigh advantages and disadvantages of long-term medications.
Methods
A literature review was conducted using keywords related to acceptance, perceptions, motivations, and barriers linked to treatment. Exploratory interviews were performed with five pharmacists and 19 patients. Interviews were systematically analyzed in order to complete the initial conceptual model. Questionnaire items were generated for each concept identified, using patients’ words. The resulting test version was tested for relevance and comprehension with six patients and revised accordingly; the new version was tested on a second set of five patients and revised to create the pilot version of the questionnaire.
Results
Items generated for each concept identified were organized into six domains: drug characteristics, duration, constraints, side effects, efficacy, and global acceptance of treatment. Except for a few items that were modified or deleted following patients’ suggestions and some minor modifications in the answer choices, the questionnaire was globally well accepted, easy to complete, and considered relevant and appropriate by patients. The pilot version of the ACCEPT© questionnaire contains 32 questions divided into the same six domains as the test version.
Conclusions
The existence of the hypothesized concept of medication acceptance was confirmed. The ACCEPT© questionnaire will allow assessment of the acceptance of a wide range of long-term medications based on patient experience. Further study will examine how well this measure predicts and explains adherence to these medications.




Similar content being viewed by others
References
World Health Organization. Adherence to long-term therapies: evidence for action [online]. Available from URL: http://www.who.int/chp/knowledge/publications/adherencereport/en/ [Accessed 2010 Jun 23]
Horne R. Non-adherence to medication: causes and implications for care. In: Gard PR, editor. A behavioural approach to pharmacy practice. Malden (MA): Blackwell Science, 2000: 111–30
Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional non-adherers, and intentional non-adherers: application of the Necessity-Concerns Framework. J Psychosom Res 2008 Jan; 64(1): 41–6
DiMatteo MR, Sherbourne CD, Hays RD, et al. Physicians’ characteristics influence patients’ adherence to medical treatment: results from the Medical Outcomes Study. Health Psychol 1993 Mar; 12(2): 93–102
DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence: a meta-analysis. Med Care 2007 Jun; 45(6): 521–8
Zolnierek KB, DiMatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care 2009 Aug; 47(8): 826–34
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005 Aug; 353(5): 487–97
Bissonnette JM. Adherence: a concept analysis. J Adv Nurs 2008 Sep; 63(6): 634–43
Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986 Jan; 24(1): 67–74
Rosenstock IM. The Health Belief Model and preventive health behavior. Health Educ Monogr 1974; 2: 354–86
Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999 Dec; 47(6): 555–67
Neame R, Hammond A. Beliefs about medications: a questionnaire survey of people with rheumatoid arthritis. Rheumatology (Oxford) 2005 Jun; 44(6): 762–7
Horne R, Weinman J, Hankins M. The Beliefs about Medicines Questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999; 14: 1–24
Kumar RN, Kirking DM, Hass SL, et al. The association of consumer expectations, experiences and satisfaction with newly prescribed medications. Qual Life Res 2007 Sep; 16(7): 1127–36
Ruiz MA, Pardo A, Rejas J, et al. Development and validation of the ‘Treatment Satisfaction with Medicines Questionnaire’ (SATMED-Q). Value Health 2008 Sep; 11(5): 913–26
Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004 Feb; 2: 12
Benson J, Britten N. What effects do patients feel from their antihypertensive tablets and how do they react to them? Qualitative analysis of interviews with patients. Fam Pract 2006 Feb; 23(1): 80–7
French LM, Smith MA, Holtrop JS, et al. Hormone therapy after the Women’s Health Initiative: a qualitative study. BMC Fam Pract 2006; 7: 61
Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 2003 Dec; 106(3): 337–45
Johnson MJ. The Medication Adherence Model: a guide for assessing medication taking. Res Theory Nurs Pract 2002; 16(3): 179–92
Patrick DL, Burke LB, Powers JH, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health 2007 Nov; 10 Suppl. 2: S125–37
Gray JR, Leung E, Scales J. Treatment of ulcerative colitis from the patient’s perspective: a survey of preferences and satisfaction with therapy. Aliment Pharmacol Ther 2009 May; 29(10): 1114–20
Lancsar EJ, Hall JP, King M, et al. Using discrete choice experiments to investigate subject preferences for preventive asthma medication. Respirology 2007 Jan; 12(1): 127–36
Stone VE, Jordan J, Tolson J, et al. Perspectives on adherence and simplicity for HIV-infected patients on antiretroviral therapy: self-report of the relative importance of multiple attributes of highly active antiretroviral therapy (HAART) regimens in predicting adherence. J Acquir Immune Defic Syndr 2004 Jul; 36(3): 808–16
Oliver RL. Satisfaction: a behavioral perspective on the consumer. New York: McGraw-Hill, 1997
Acknowledgments
The authors would like to thank Sabrina Ranchon (MAPI Consultancy) for her help throughout the development process. They would also like to thank the MAPI Research Trust for conducting the literature search. They thank Isabelle Guillemin from MAPI Consultancy for reviewing the manuscript, Anne Brédart from Institut Curiefor helping draft and review the manuscript, and Sara Strzok (Minneapolis, MN, USA)for reviewing and editing the manuscript for English language.
Financial support for this study was provided by Registrat-MAPI.
C. Marant, B. Arnould, C. Spizak, A. Marrel and D. Patrick were paid consultants to Registrat-MAPI. R. Gauchoux is an employee of Registrat-MAPI and J. Longin was an employee of Registrat-MAPI. E. Van Ganse has no conflicts of interest to declare.
Copyright
The ACCEPT© questionnaire is protected by copyright with all rights reserved to Registrat-MAPI. Do not use this questionnaire without permission. For information on or permission to use the ACCEPT© questionnaire, please contact the MAPI Research Trust, 27 rue de la Villette, 69003 Lyon, France. Telephone: +33 (0)472 13 65 75; e-mail: www.trust@mapi.fr; website: http://www.mapi-trust.org.
Author contributions
C. Marant participated in the pharmacist and patient interviews, and contributed to the data analysis and interpretation, and writing of the manuscript. J. Longin contributed to the study concept and design, data interpretation, and reviewing of the manuscript. R. Gauchoux contributed to the study concept and design, data interpretation, and reviewing of the manuscript. B. Arnould contributed to the study concept and design, data interpretation, and reviewing of the manuscript. C. Spizak participated in the patient interviews, and contributed to data analysis and interpretation, and reviewing of the manuscript. A. Marrel contributed to the study concept and design, data interpretation, and reviewing of the manuscript. D. Patrick contributed to the study concept and design, data interpretation, and reviewing of the manuscript. E. Van Ganse contributed to the study concept and design, data interpretation, and reviewing of the manuscript. B. Arnould is the guarantor for the overall content of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Marant, C., Longin, J., Gauchoux, R. et al. Long-Term Treatment Acceptance. Patient-Patient-Centered-Outcome-Res 5, 239–249 (2012). https://doi.org/10.1007/BF03262496
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03262496