Abstract
Background: PCR-based detection of microorganisms is widely used for diagnostic purposes. Most routine PCR applications do not control for inhibition of PCR, thus leading to false-negative results.
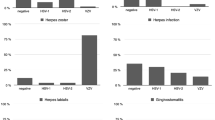
Methods and Results: One hundred eighteen swab samples obtained from skin and mucosa were investigated for the presence of herpes simplex virus (HSV), varicellazoster virus (VZV), and the control gene β-globin by internally controlled PCR with purified and unpurified DNA in parallel. With unpurified DNA, inhibition of PCR was detected in 23% of β-globin PCRs, 25% of VZV PCRs, and 16% of HSV PCRs versus 3% each for purified DNA. Approximately 20% of the samples with positive results for HSV or VZV had negative or inhibited results using unpurified DNA.
Conclusion: These results indicate that PCR from clinical swab specimens should be performed exclusively with internal controls because the positive control alone cannot exclude PCR inhibition in individual samples. Purification of DNA will decrease, but not exclude, PCR inhibition.
Similar content being viewed by others
References
Verkooyen RP, Luijendijk A, Huisman WM, et al.: Detection of PCR inhibitors in cervical specimens by using the Amplicor Chlamydia trachomatis assay. J Clin Microbiol 1996;34:3072–3074
Rübben A, Baron JM, Grussendorf-Conen EI: Routine detection of herpes simplex virus and varicella zoster virus by polymerase chain reaction reveals that initial herpes zoster is frequently misdiagnosed as herpes simplex. Br J Dermatol 1997;137:259–261
Nahass GT, Mandel MJ, Cook S, Fan W, Leonardi CL: Detection of herpes simplex infection from cutaneous lesions in different clinical stages with the polymerase chain reaction. J Am Acad Dermatol 1995;32:730–733
Cone RW, Hobson AC, Palmer J, Remington M, Corey L: Extended duration of herpes simplex virus DNA in genital lesions detected by the polymerase chain reaction. J Infect Dis 1991;164:757–760
LaRussa P, Steinberg S, Meurice F, Gershon A: Transmission of vaccine strain varicella-zoster virus from a healthy adult with vaccine-associated rash to susceptible household contacts. J Infect Dis 1997;176:1072–1075
Jackson R, Morris DJ, Cooper RJ, et al.: Multiplex polymerase chain reaction for adenovirus and herpes simplex virus in eye swabs. J Virol Methods 1996;56:41–48
Furuta Y, Fukuda S, Suzuki S, Takasu T, Inuyama Y, Nagashima K: Detection of varicella-zoster virus DNA in patients with acute peripheral facial palsy by the polymerase chain reaction, and its use for early diagnosis of zoster sine herpete. J Med Virol 1997;52:316–319
Lichtensteiger CA, Steenbergen SM, Lee RM, Polson DD, Vimr ER: Direct PCR analysis for toxigenic Pasteurella multocida. J Clin Microbiol 1996;34:3035–3039
Saiki RK, Gelfand DH, Stoffel S, et al.: Primer directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988;239:487–491
Cao M, Xiao X, Egbert B, Darragh TM, Yen TSB: Rapid detection of cutaneous herpes simplex virus infection with the polymerase chain reaction. J Invest Dermatol 1989;82:391–392
Tsurumi T, Maeno K, Nishigama Y: Nucleotide sequence of the DNA polymerase gene of herpes simplex virus type 2 and comparison with the type 1 counterpart. Gene 1987;52:129–137
Puchhammer-Stöckl E, Popow-Kraupp T, Heinz FX, Mandl CW, Kunz C: Detection of varicella-zoster virus DNA by polymerase chain reaction in the cerebrospinal fluid of patients suffering from neurological complications associated with chicken pox or herpes zoster. J Clin Microbiol 1991;29:1513–1516
Saiki RK, Scharf S, Faloona F, et al.: Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 1985;230:1350–1354
Siebert PD, Larrick JW: Competitive PCR. Nature 1992;359:557–558
Cone RW, Hobson AC, Huang MLW: Coamplified positive control detects inhibition of polymerase chain reaction. J Clin Microbiol 1992;30:3185–3189
Zerbini M, Gallinella G, Manaresi E, Musiani M, Gentilomi G, Venturoli S: Standardization of a PCR ELISA in serum samples: Diagnosis of active parvovirus B19 infection. J Med Virol 1999;59:239–244
Thoreson AC, Borre M, Andersen LP, et al.: Helicobacter pylori detection in human biopsies: A competitive PCR assay with internal control reveals false results. FEMS Immunol Med Microbiol 1999;24:201–208
Lee PY, Mangan J, Holliman RE, Butcher PD: Quantitation of Toxoplasma gondii DNA in a competitive nested polymerase chain reaction. J Clin Pathol 1999;52:61–64
Lichtinghagen R, Glaubitz R: A competitive polymerase chain reaction assay for reliable identification of Bordetella pertussis in nasopharyngeal swabs. Eur J Clin Chem Clin Biochem 1995;33:87–93
Hohlfeld P, Daffos F, Costa JM, Thulliez P, Forestier F, Vidaud M: Prenatal diagnosis of congenital toxoplasmosis with a polymerase chain reaction test on amniotic fluid. N Engl J Med 1994;331:695–699
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bezold, G., Volkenandt, M., GottlÖber, P. et al. Detection of Herpes Simplex Virus and Varicella- zoster Virus in Clinical Swabs: Frequent Inhibition of PCR as Determined by Internal Controls. Molecular Diagnosis 5, 279–284 (2000). https://doi.org/10.1007/BF03262088
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03262088