Abstract
Background: Acute extrapyramidal reactions have been attributed to amodiaquine. They may be anticipated with the widely-used combination anti-malarial artesunate with amodiaquine, but the association is very poorly documented.
Objective: The aim of the study was to identify individual case safety reports in the Uppsala Monitoring Centre’s Vigibase™ database associating the use of the combination of artesunate and amodiaquine with extrapyramidal adverse reactions and to characterize the clinical features in those reports.
Methods: Reports of adverse reactions to the combination use of artesunate or dihydroartemisinin and amodiaquine entered into Vigibase™ up to 15 February 2011 were identified. Reports with a causality grading of ‘Unlikely’ and probable duplicates of reports were excluded. Reports that included at least one MedDRA® Preferred Term strongly suggestive of an extrapyramidal reaction were subject to further detailed analysis.
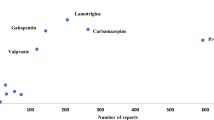
Results: Forty-three reports in adults and six reports in children were identified as associating the use of artesunate with amodiaquine, either as separate co-packaged or fixed-combination products, with extrapyramidal reactions. More than half (57%) of the adults had an onset of the reaction within 48 hours of starting treatment. Almost equal numbers of male and female adult patients were reported — 67% were aged between 14 and 30 years. The most commonly implicated daily dose was amodiaquine base 600 mg and artesunate 200 mg, but lower doses were implicated in some adult patients. Identification of very long delays in some reports reaching Vigibase™ was an unexpected observation.
Conclusions: This case series supports an association of the use of artesunate and amodiaquine as combination antimalarial therapy with acute extra-pyramidal reactions. The reactions occurred with recommended, and in some instances reduced, daily doses. Extrapyramidal reactions are unpleasant and frightening and the association warrants being more clearly recorded in official treatment guidelines and Summary of Product Characteristics documents.





Similar content being viewed by others
References
Akindele MO, Odejide AO. Amodiaquine-induced involuntary movements. BMJ 1976; 2: 14–5
Adonis-Koffy L, Daubrey T, Kouadio A, et al. Effets neurologiques indésirables méconnus de l’amodiaquine. Bull Soc Pathol Exot 2003; 96: 302–7
Kamagaté M, Dié-Kacou H, Balayssac E, et al. Oro-facial dyskinesias and amodiaquine [letter]. Therapie 2004; 59: 565–6
Akpalu AK, Nyame PK, Dodoo ANO. Amodiaquine-induced dystonic reactions: case reports and implications for policy change in Ghana. Int J Risk Safety Medicine 2005; 17: 1–4
World Health Organization. Guidelines for the treatment of malaria. 2nd ed. Geneva: World Health Organization, 2010: ix
Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inf J 2008; 42: 409–19
Introductory guide to standardised MedDRA queries (SMQs). Version 14.0. Chantilly (VA). Maintenance and Support Services Organization, 2011 Mar
Dodoo ANO. Global support for regional problems? Uppsala Reports 2008; 43: 13 [online]. Available from URL: http://www.who-umc.org/graphics/24357.pdf [Accessed 2010 Jun 16]
Fajolu IB, Lesi FEA. Drug-induced acute dystonia in a 7 year old child following the use of artesunate-amodiaquine: a case report. Int J Risk Saf Med 2010; 22: 111–4
Ball R. Drug-induced akathisia: a review. J R Soc Med 1985; 78: 748–52
Bateman DN, Rawlins MD, Simpson JM. Extrapyramidal reactions with metoclopramide. BMJ 1985; 291: 930–2
Parikh S, Ouedraogo JB, Goldstein JA, et al. Amodiaquine metabolism is impaired by common polymorphisms in CYP2C8: implications for malaria treatment in Africa. Clin Pharmacol Ther 2007; 82: 197–203
Gil JP. Amodiaquine pharmacogenetics. Pharmacogenomics 2008; 9: 1385–90
World Health Organization. Guidelines for the treatment of malaria. Geneva: World Health Organization, 2006: 89
World Health Organization. Guidelines for the treatment of malaria. 2nd ed. Geneva: World Health Organization, 2010: 75
Taylor WRJ, Terlouw DJ, Olliaro PL, et al. Use of weight-for-age-data to optimize tablet strength and dosing regimens for a new fixed-dose artesunate-amodiaquine combination for treating falciparum malaria. Bull World Health Organ 2006; 84: 956–64
Staedke SG, Jagannathan P, Yeka A, et al. Monitoring antimalarial safety and tolerability in clinical trials: a case study from Uganda. Malar J 2008; 7: 107–17
Sinclair D, Zani B, Donegan S, et al. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev 2009; (3): CD007483
Summary of product characteristics: artesunate/amodiaquine tablets (Sanofi-Aventis), MA056, MA057, MA058. In: WHOPAR part 4. 09/2010, version 1.1: 6-7 [online]. Available from URL: http://apps.who.int/prequal/WHOPAR/WHOPARPRODUCTS/MA058part4v1.pdf [Accessed 2011 May 30]
Sanofi-Aventis. Application for inclusion of artesunate/amodiaquine fixed dose combination tablets in the WHO model lists of essential medicines. June 2009, updated Sept 2010 [online]. Available from URL: http://www.who.int/selection_medicines/committees/expert/18/applications/Sanofi_application.pdf [Accessed 2011 Mar 28]
Summary of product characteristics: amodiaquine (150mg base) tablets (Guilin), MA045. In: WHOPAR part 4. 06/2011 [online]. Available from URL: http://apps.who.int/prequal/WHOPAR/WHOPARPRODUCTS/MA045part4v2.pdf [Accessed 2011 Sept 15]
Caveat document: accompanying statement to data released from the WHO Collaborating Centre. WHO Collaborating Centre for International Drug Monitoring [online]. Available from URL: http://www.who-umc.org/graphics/25027.pdf [Accessed 2011 Jun 19]
WHO briefing on malaria treatment guidelines and artemisinin monotherapies. Geneva: World Health Organization, 2006: 17 [online]. Available from URL: http://www.who.int/malaria/publications/atoz/meeting_briefing19april.pdf [Accessed 2011 May 19]
Talisuna AO, Staedke SG, D’Alessandro U. Pharmacovigilance of antimalarial treatment in Africa: is it possible? Malar J 2006; 5: 50–8
Acknowledgements
Marie Lindquist (Director, UMC), Ralph Edwards (Former Director, UMC) and Alex Dodoo (Director, WHO Collaborating Centre for Advocacy and Training in Pharmacovigilance, Accra, Ghana) encouraged the author to undertake the review. The WHO Collaborating Centre for International Drug Monitoring has authorized the use and publication of the VigiBase™ data. Alex Dodoo and Mimi Darko (Food and Drugs Board, Ghana) readily answered many questions. Richard Hill and Sara Hult (UMC) assisted greatly with searches of Vigibase, and Monica Ploen resolved some data retrieval difficulties identified during the study. The author presented key data from this paper in the Bengt-Erik Wiholm Memorial Lecture, 11th Annual Meeting, International Society of Pharmacovigilance, Istanbul, Turkey, 27 October 2011.
There was no funding for the study. John McEwen proposed the study, undertook the searches of VigiBase™, analysed the data and prepared the report. He undertook the study in his free time. He certifies that he has had full access to all the data in the study and decided to submit the paper for publication.
John McEwen is a volunteer (unpaid) member of a panel of reviewers of data for the UMC. He had a short-term contract with the WHO in 2006 to provide an expert review of reports of suspected reactions to artesunate with amodiaquine in Ghana. He is an (unpaid) Adjunct Associate Professor, Discipline of Pharmacy, University of Canberra, Canberra, ACT, Australia. He is a part-time Medical Adviser, Therapeutic Goods Administration, Australia, contracted until May 2013, and has undertaken paid evaluations of medicines for the Australian Department of Health and Ageing. He has, in the past 4 years, undertaken paid expert evaluations for the WHO of vaccines proposed for pre-qualification. He has had travel expenses reimbursed, accommodation reimbursed and in some instances been paid a per diem fee or a gratuity to give pharmacovigilance training for the International Society of Pharmacovigilance, the Chinese University of Hong Kong, Health Sciences Authority Singapore, IBC Asia (S) Pte Ltd and the Government of Indonesia, and to review pharmacovigilance arrangements for the Governments of Hong Kong and Vanuatu. In 2010, at the request of the WHO, he gave unpaid advice about pharmacovigilance of an antimalarial drug (not artesunate with amodiaquine) to the European Medicines Agency.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McEwen, J. Artesunate- and Amodiaquine-Associated Extrapyramidal Reactions. Drug Saf 35, 667–675 (2012). https://doi.org/10.1007/BF03261963
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03261963