Abstract
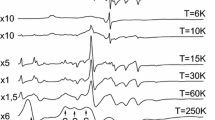
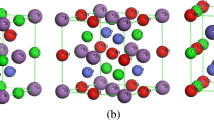
Ca2CuO3 is an essentially diamagnetic oxide, in spite of its EPR spectrum. The latter is similar to literature spectra attributed to dimers or clusters formed by copper ions. A model based on the formation of triplet-state defects involving two neighbor copper ions is then discussed to explain these experimental observations. These defects would also propagate in the crystal and interact with each other.
Similar content being viewed by others
References
Von Teske Chr.L., Mueller-Buschbaurn Hk.: Zeitschrift für Anorg. Allgem. Chemie 379, 234–241 (1970)
Wasserman E., Snyder L.C., Yager W.A.: J. Chem. Phys. 41, 1763–1772 (1964)
Wertz J.E., Bolton J.R.: Electron Spin Resonance. Elementary Theory and Practical Applications, p.223. New York: Mc Graw-Hill 1972.
Atherton N.M.: Electron Spin Resonance. Theory and Applications, p.150. New York: J.Wiley 1973.
Chesnut D.B., Phillips W.D.:J. Chem. Phys. 35, 1002–1012 (1961)
Sternlicht H., McConnell H.M.: J. Chem. Phys. 35, 1793 (1961)
McConnel H.M., Lynden-Bell R.: J. Chem. Phys. 36, 2393–2397 (1962)
Barnes J.A., Hodgson D.J., Hatfield W.E.: Inorg. Chem. 11, 144–148 (1972)
Kohout J., Liska M., Hvastijova’ M.: Inorg. Chim. Acta 25, L 71–L 73 (1977)
Dickinson R.C., Helm F.T., Baker W.A. Jr., Black T.D., Watson W.H. Jr.: Inorg. Chem. 16, 1530–1537 (1977)
Wasson J.R., Shyr C.I., Trapp C.: Inorg. Chem. 7, 469–473 (1968)
Kreiter A., Huettermann J.: J. Magn. Reson. 93, 12–26 (1991)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oliva, C., Forni, L., Ponti, A. et al. EPR Study of Triplet Defects in Ca2CuO3 . Appl Magn Reson 3, 873–881 (1992). https://doi.org/10.1007/BF03260118
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03260118