Summary
To improve the biopharmaceutical performance of the nonsteroidal anti-inflammatory drug piroxicam, inclusion compounds with β-cyclodextrin carrier have been prepared and studied for a wide range of chemical, physical and pharmaceutical properties.
Three bulk products were obtained, 2 by a process of freeze-drying (preparation A/1 and preparation A/2) and changing process parameters, and 1 by spray-drying (preparation B). The objective was to obtain tablets which provide a rapid-release dosage form of piroxicam.
Studies were conducted in vitro and in humans and compared tablets of the inclusion complex of piroxicam and β-cyclodextrin with marketed oral and parenteral dosage forms of piroxicam. Significant differences were found in plasma levels, maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC), confirming that biopharmaceutical optimisation of the complexes can be achieved, leading to an overall pattern of absorption which is better than those presented by standard, non-complexed formulations of piroxicam (capsules, and ampoules for intramuscular use).
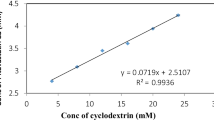
Evidence for a correlation between the 2 main physicopharmaceutical variables (solubilisation kinetics of the complex and dissolution rate of the tablet) and biopharmaceutical performances support the development of the product.
Similar content being viewed by others
References
Boscolo M, Carli F, Crimella T, et al. Evaluation on the suitability of differential scanning calorimetry in the preformulation stability screening. Drug Development and Industrial Pharmacy, in press
Carli F, Motta A. Particle size and surface area distribution of pharmaceutical powders by microcomputizer mercury porosimetry Journal of Pharmaceutical Sciences 73: 197, 1984
Cassie ABO, Baxter S. Transactions of the Faraday Society 40: 546, 1944
Eatough DJ, Christensen JJ, Izatt RM. Experiments in thermometric titrimetry and titration calorimetry. Brigham Young University Press, Provo, Utah, 1973
Elgezawi S, Omar N, Elrabbat N, Ueda H. Microcalorimetric and chromatographic investigation of the binding of some pyridine derivatives of cyclodextrins. Journal of Pharmaceutical and Biomédical Analysis 4: 399–406, 1988
Higuchi T, Connors K. Phase-solubility techniques. Advances in Analytical Chemistry and Instrumentation 4: 117–212, 1965
Kurozumi M, Nambu N, Nagai T. Inclusion compounds of nonsteroidal antiinflammatory and other slightly water soluble drugs with alpha and beta cyclodextrins in powdered form. Chemical and Pharmaceutical Bulletin 23: 3062–3068, 1975
Lewis EA, Hansen LD. Thermodynamics of binding of guest molecules to alpha- and beta-cyclodextrins. Journal of the Chemical Society. Perkin Trans. 2: 2081–2085, 1973
Mack GL. Journal of Physical Chemistry 40: 159, 1936
Mihalic M, Hofman H, Kajfez F, Kuftinec J. Blazevic N, Zinic M. Physico-chemical and analytical characteristic of piroxicam. Acta Pharmaceutica Jugoslavica 32: 13–20, 1982
Patoia L, Clausi G, Farroni F, Alberti P, Fugiani P, et al. Faecal blood loss, upper gastro-intestinal mucosal integrity and symptoms following piroxicam β-cyclodextrin, piroxicam and placebo administration: a comparative study. European Journal of Clinical Pharmacology, in press
Richardson CJ, Ross SG, Bloka K, Verbeeck RK. High-performance liquid chromatographic analysis of piroxicam and its major metabolite 5′-hydroxypiroxicam in human plasma and urine. Journal of Chromatography 382: 382–388, 1986
Roos-Hoffet A. Characterization of β-cyclodextrin complexes. Proceeding of the 4th International Symposium on Cyclodextrins, Kluwer Academic Publishers, Dordrecht, 1988
Szeijtli J. Cyclodextrins and their inclusion complexes, Academiai Kiado, Budapest, 1982
Tokomura T, Tsushima Y, Tatsuishi K, Kayano M, Nachida Y, et al. Preparation of cinnarrizine/β-cyclodextrin inclusion complex by spray-drying method and the stability of complex in solid state. Yakuzaigaku 45: 1–6, 1985
Uekama K, Ikeda Y, Hirayama F, Otagiri M, Shibata M. Inclusion complexation of hydroxybenzoic acid esters with alpha and beta cyclodextrins: dissolution behaviours and antimicrobial activities, Yakugaku Zasshi 100: 994, 1980
Uekama K, Otagiri M. Cyclodextrins in drug carrier systems. CRC Critical Reviews in Therapeutic Drug Carrier Systems 3: 1–40, 1987
Wiseman EH, Chang YH, Lombardino JC. Piroxicam, a novel anti-inflammatory agent. Arzneimittel-Forschung 26: 1300–1303, 1976
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Acerbi, D., Bovis, G., Carli, F. et al. Biopharmaceutical Optimisation of β-Cyclodextrin Inclusion Compounds. Drug Invest 2 (Suppl 4), 29–36 (1990). https://doi.org/10.1007/BF03258224
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03258224