Summary
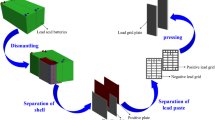
Previous work at the Bureau of Mines Rolla Research Center, U.S. Department of the Interior, resulted in successful development of a bench-scale, combination electrorefining-electrowinning method for recycling lead from scrap batteries by using waste fluosilicic acid (H2SiF6) as electrolyte.1,2 This paper describes larger scale experiments. Prior attempts to electrowin lead failed because large quantities of insoluble lead dioxide were deposited on the anodes at the expense of lead deposition on the cathodes. A major breakthrough was achieved with the discovery that lead dioxide formation at the anodes is prevented by adding a small amount of phosphorus to the electrolyte. The amount of PbO2 formed on the anodes during lead electrowinning was less than 1% of the total lead deposited on the cathodes. This work recently won the prestigious IR·100 award as one of the 100 most significant technological advances of 1984.
Similar content being viewed by others
References
E. R. Cole, A. Y. Lee, and D. L. Paulson, “Electrolytic Method for Recovery of Lead From Scrap Batteries,” U.S. Department of the Interior, Bureau of Mines, Report of Investigations 8602, (1981).
E. R. Cole, A. Y. Lee, and D. L. Paulson, “Recovery of Lead From Battery Sludge by Electrowinning,” Journal of Metals, 35(8), (1983), pp. 42–46.
T. A. Phillips, “Preliminary Economic and Technical Evaluation of an Electrolytic Process to Recovery Lead From Scrap Batteries,” BuMines, Nov. 1981, 23 pp. Available for consultation at the Rolla Research Center, Bureau of Mines, Rolla, Mo.
L. L. Smith, R. G. Sandberg, and E. R. Cole, U. S. Department of the Interior, U.S. Patent No. 4,159,231, June 16, 1979.
A. Y. Lee, E. R. Cole, Jr., and D. L. Paulson, “Electrolytic Method for Recovery of Lead From Scrap Batteries: Scale-Up Study Using 20-Liter Multielectrode Cell,” U.S. Department of the Interior, Bureau of Mines, Report of Investigations 8857, (1984).
A. Y. Lee, E. R. Cole, Jr., and D. L. Paulson, “Electrorefining Missouri Low-Antimony Lead Bullion,” U.S. Department of the Interior, Bureau of Mines, Report of Investigations 8401, (1979).
D. L. Thomas, C. J. Krauss, and R. C. Kerby, “Betts Lead Electrorefining at Cominco,” TMS paper selection A81-8.
R. G. Prengaman and H. B. McDonald, RSR Corporation, U.S. Patent No. 4,230,545, October 28, 1980.
J. B. Weaver and H. C, Bauman, “Cost and Profitability Estimation,” in Perry’s Chemical Engineers’ Handbook, ed. by R. H. Perry and C. H. Chilton, McGraw-Hill, 5th ed., 1973, pp. 25-45.
Additional information
Editor’s Note: The original bench scale testing was reported in the August 1983 Journal of Metals.
Dr. E. R. Cole received his Ph.D. (1970) in metallurgical engineering from the Uni-versity of Missouri-Rolla. He is currently a Research Supervisor at the U.S. Bureau of Mines, Rolla Research Center in Rolla, Missouri.
Dan L. Paulson is a Chemical Engineer at the U.S. Bureau of Mines, Rolla Research Center in Rolla, Missouri.
Agnes V. Lee is Research Director at the U.S. Bureau of Mines, Rolla Research Center in Rolla, Missouri.
Rights and permissions
About this article
Cite this article
Cole, E.R., Lee, A.Y. & Paulson, D.L. Update on Recovering Lead From Scrap Batteries. JOM 37, 79–83 (1985). https://doi.org/10.1007/BF03257768
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03257768