Abstract
Background: With 1.8 million new cases each year, India carries 20% of the global burden of tuberculosis, a situation that is now further exacerbated with the emergence of drug resistance. The current diagnostic technique suggested by the Government of India’s Revised National Tuberculosis Control Programme is Ziehl-Neelsen staining of a sputum smear. This technique is known to be inadequate.
Objective: The aim of this study was to evaluate nested PCR (nPCR) in the detection of pulmonary tuberculosis in sputum samples in comparison with conventional smear findings, in an effort to improve detection rates from those obtained by the smear-alone approach.
Study Design: Patients attending a tertiary-care hospital (situated in a rural area of Vellore district) with clinical suspicion of pulmonary tuberculosis were prospectively recruited from mid-April 2009 to mid-December 2009 and investigated. The sputum samples were stained by Ziehl-Neelsen staining for smear examination. DNA extracted from concentrated sputum was tested by nPCR, targeting the IS6110 sequence in the Mycobacterium tuberculosis genome.
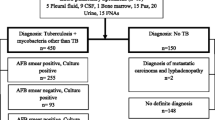
Results: Among 84 patients tested (median age 45.5 years), 80.95% were from the rural community and 19.05% were from the peri-urban community. Seventeen patients (20.24%; mid-p 95% CI 31.5, 52.4) tested positive by the smear examination and 35 (41.67%; mid-p 95% CI 12.7, 29.8) tested positive by nPCR. The difference in detection rates was statistically significant (χ2 = 9.02; p = 0.002). The κ coefficient between smear findings and nPCR findings was 0.47, which was a statistically significant agreement (Z = 4.91; p<0.0001).
Conclusion: This report describes the molecular detection of M. tuberculosis in patients’ sputum samples tested by the nPCR format, using IS6110 as a target sequence. A high prevalence of pulmonary tuberculosis was identified by the nPCR assay, which was shown to have a significantly higher detection rate than conventional smear staining.


Similar content being viewed by others
References
World Health Organization. Country profile: India. In: WHO report 2009: global tuberculosis control — epidemiology, strategy, financing [WHO report no. WHO/HTM/TB/2009.411]. Geneva: WHO, 2009: 109-12 [online]. Available from URL: http://www.who.int/tb/publications/global_report/2009/en/index.html [Accessed 2010 May 28]
Jaggarajamma K, Sudha G, Chandrasekaran V, et al. Reasons for non-compliance among patients treated under Revised National Tuberculosis Control Programme (RNTCP), Tiruvallur District, South India. Indian J Tuberc 2007; 54: 130–5
Aslanzadeh J, de la Viuda M, Fille M,et al. Comparison of culture and acid-fast bacilli stain to PCR for detection of Mycobacterium tuberculosis in clinical samples. Mol Cell Probes 1998; 12: 207–11
Nolte FS, Metchock B, McGowan JE, et al. Direct detection of Mycobacterium tuberculosis in sputum by polymerase chain reaction and DNA hybridization. J Clin Microbiol 1993; 31: 1777–82
Kocagöz T, Yilmaz E, Ozkara S, et al. Detection of Mycobacterium tuberculosis in sputum samples by polymerase chain reaction using a simplified procedure. J Clin Microbiol 1993; 31: 1435–8
García-Quintanilla A, Garcia L, Tudó G, et al. Single-tube balanced heminested PCR for detecting Mycobacterium tuberculosis in smear-negative samples. J Clin Microbiol 2000; 38: 1166–9
Gengvinij N, Pattanakitsakul SN, Chierakul N, et al. Detection of Mycobacterium tuberculosis from sputum specimens using one-tube nested PCR. Southeast Asian J Trop Med Public Health 2001; 32: 114–25
García-Quintanilla A, González-Martín J, Tudó G, et al. Simultaneous identification of Mycobacterium genus and Mycobacterium tuberculosis complex in clinical samples by 5′-exonuclease fluorogenic PCR. J Clin Microbiol 2002; 40: 4646–51
Lemaître N, Armand S, Vachée A, et al. Comparison of the real-time PCR method and the Gen-Probe amplified Mycobacterium tuberculosis direct test for detection of Mycobacterium tuberculosis in pulmonary and non-pulmonary specimens. J Clin Microbiol 2004; 42: 4307–9
Takahashi T, Nakayama T. Novel technique of quantitative nested real-time PCR assay for Mycobacterium tuberculosis DNA. J Clin Microbiol 2006; 44: 1029–39
De Beenhouwerc H, Lhiang Z, Jannes G, et al. Rapid detection of rifampicin resistance in sputum and biopsy specimens from tuberculosis patients by PCR and line probe assay. Tuber Lung Dis 1995; 76(5): 425–30
Nandagopal B, Sankar S, Karthikeyan L, et al. Evaluation of a nested PCR targeting IS6110 of Mycobacterium tuberculosis for the detection of the organism in the leukocyte fraction of blood samples. Indian J Med Microbiol. In press
Lima JF, Montenegro LM, Montenegro RA, et al. Performance of nested PCR in the specific detection of Mycobacterium tuberculosis complex in blood samples of pediatric patients. J Bras Pneumol 2009; 35: 690–7
Ayele WY, Bartos M, Svastova P, et al. Distribution of Mycobacterium avium subsp. paratuberculosis in organs of naturally infected bull-calves and breeding bulls. Vet Microbiol 2004; 103: 209–17
Davis JL, Huang L, Kovacs JA, et al. Polymerase chain reaction of secA1 on sputum or oral wash samples for the diagnosis of pulmonary tuberculosis. Clin Infect Dis 2009; 48(6): 725–32
Omrani M, Ansari MH, Agaverdizadae D. PCR and ELISA methods (IgG and IgM): their comparison with conventional techniques for diagnosis of Mycobacterium tuberculosis. Pak J Biol Sci 2009; 12(4): 373–7
Narayanan S, Das S, Garg R, et al. Molecular epidemiology of tuberculosis in a rural area of high prevalence in South India: implications for disease control and prevention. J Clin Microbiol 2002; 40: 4785–8
Djelouagji Z, Drancourt M. Inactivation of cultured Mycobacterium tuberculosis organisms prior to DNA extraction. J Clin Microbiol 2006; 44: 1594–5
Hosek J, Svastova P, Moravkova M, et al. Methods of mycobacterial DNA isolation from different biological material: a review. Veterinarni Medicina 2006; 51: 180–92
Petroff SA. A new and rapid method for the isolation and cultivation of tubercle bacilli directly from the sputum and feces. J Exp Med 1915; 21: 38–42
Carricajo A, Fonsale N, Vautrin AC, et al. Evaluation of BacT/Alert 3D liquid culture system for recovery of mycobacteria from clinical specimens using sodium dodecyl (lauryl) sulfate-NaOH decontamination. J Clin Microbiol 2001; 39: 3799–800
Lakshmi V, Patil MA, Subhadha K, et al. Isolation of mycobacteria by Bactec 460 TB system from clinical specimens. Ind J Med Microbiol 2006; 24: 124–6
Scarparo C, Piccoli P, Rigon A, et al. Direct identification of mycobacteria from MB/BacT Alert 3D bottles: comparative evaluation of two commercial probe assays. J Clin Microbiol 2001; 39: 3222–7
Acknowledgments
The research work was funded by an intramural research support fund, which is gratefully acknowledged. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sankar, S., Balakrishnan, B., Nandagopal, B. et al. Comparative Evaluation of Nested PCR and Conventional Smear Methods for the Detection of Mycobacterium tuberculosis in Sputum Samples. Mol Diag Ther 14, 223–227 (2010). https://doi.org/10.1007/BF03256377
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256377