Abstract
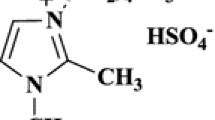
Brønsted acidic ionic liquid, triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate, has been used as an efficient and reusable catalyst for the one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles under solvent-free conditions in excellent yields.
Similar content being viewed by others
References
M.M. Heravi, S. Sadjadi, J. Iran. Chem. Soc. 6 (2009) 1.
J. Zhu, H. Bienayme, Multicomponent Reaction, Wily- VCH, France, 2005.
K. Niknam, A. Fatehi-Raviz, J. Iran. Chem. Soc. 4 (2007) 438
B. Sadeghi, B.B.F. Mirjalili, S. Bidaki, M. Ghasemkhani, J. Iran. Chem. Soc. 8 (2011) 648.
R. Breslow, Acc. Chem. Res. 28 (1995) 146.
H.V.D. Bossche, G. Willemsens, W. Cools, P. Marichal, W. Lauwers, Bio. Chem. Soc. Trans. 11 (1983) 665.
J. Freedman, J. Loscalzo, New Therapeutic Agent in Thrombosis and Thrombolysis, 3th ed., Taylor and Francis, 2009.
A.R. Hajipour, F. Rafiee, J. Iran. Chem. Soc. 6 (2009) 647.
H. Shaterian, A.R. Oveisi. J. Iran. Chem. Soc. 8 (2011) 545.
M. Freemantle, Introduction to Ionic Liquids, Royal Society of Chemistry, Cambridg CB4 OWF, UK, 2009.
P. Wasserscheid, T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Germany, 2008.
R.A. Sheldon, I. Arends, U. Hanefeld, Green Chemistry and Catalyst, Delft University of Tecnology, The Netherlands, Wiley-VCH, 2007.
H.R. Shaterian, H. Yarahmadi, M. Ghashang, Tetrahedron 64 (2008) 1263
H.R. Shaterian, M. Honarmand, A.R. Oveisi, Monatsh. Chem. 141 (2010) 557
H.R. Shaterian, A.R. Oveisi, Chin. J. Chem. 27 (2009) 2418
H.R. Shaterian, A. Hossienian, M. Ghashang, Turk. J. Chem. 2 (2009) 233.
C. Cole, J.L. Jensen, I. Nati, K.L.T. Tran, K.J. Weaver, D. Forbes, J. Am. Chem. Soc. 124 (2002) 5962.
J. Liu, J. Chem, J. Zhao, Y. Zhao, L. Li, H. Zhang, Synthesis (2003) 2661.
H. Weinmann, M. Harre, K. Koeing, E. Merten, U. Tilestam, Tetrahedron Lett. 43 (2002) 593.
S. Sarshar, D. Siev, A.M.M. Mjalli, Tetrahedron Lett. 37 (1996) 835
D.E. Frantz, L. Morency, A. Soheili, J.A. Murry, E.J.J. Grabowski, R.D. Tillyer, Org. Lett. 6 (2004) 843.
M.V. Chary, N.C. Keerthysri, S.V.N. Vupallapati, N. Lingaiahi, S. Kantevari, Catal. Commun. 9 (2008) 2013.
L.M. Wang, Y.H. Wang, H. Tian, Y.F. Yao, J.H. Shao, B. Liu, J. Fluorine Chem. 127 (2006) 1570.
S.A. Siddiqui, U.C. Narkhede, S.S. Palimkar, T. Daniel, R.J. Lahoti, K.V. Srinivasan, Tetrahedron 61 (2005) 3539.
T. Heinze, T. Liebert, Prog. Polym. Sci. 26 (2001) 1689.
D.M. White, J. Sonnenberg. J. Org. Chem. 29 (1964) 1926.
J.F. Zhou, Y.Z. Song, Y.L. Yang, Y.L. Zhu, Synth. Commun. 35 (2005) 1369.
S. Samai, G.C. Nandi, M.S. Singh, Tetrahedron 65 (2009) 10155.
M. Frank, DE Patent 4, 320, 802 (1995).
D.A. Evans, K.M. Lundy, J. Am. Chem. Soc. 114 (1992) 1495.
A. Hasaninejad, A. Zare, M. Shokouhy, J. Ameri Rad, J. Comb. Chem. 12 (2010) 844.
K.F. Shelke, S.B. Sapkal, S.S. Sonar, B. Korean. J. Am. Chem. Soc. 30 (2009) 5.
H. Zang, Q. Su, Mo. Yingming, B.W. Cheng, S. Jun, Ultrason. Sonochem. 17 (2010) 749.
R.P. Kale, G.R. Jadhav, M.U. Shaikh, Tetrahedron Lett. 50 (2009) 1780.
A. Mohammadi, M. Mivechi, H. Kefayati, Monatsh. Chem. 139 (2008) 935.
D.E. Frantz, L. Morency, A. Soheilli, J.A. Murrry, E.J.J. Grabowski, R.D. Tillyer, Org. Lett. 6 (2004) 843.
M.M. Heravi, F. Drikvand, F.F. Bamoharram, J. Mol. Catal. 263 (2007) 112.
A. Karimi, R. Alimohammadi, Z. Azizian, J. Mohammadi, A.A. Mohammadizadeh, Catal. Commun. 7 (2006) 728.
S. Kantevari, S.V.N. Vuppalapati, D.O. Biradar, L. Nagarapu, J. Mol. Catal. 266 (2007) 109.
A. Usyatinsky, Y. Khmelnitsky, Tetrahedron Lett. 41 (2000) 5031.
B. Sadeghi, B.B.F. Mirjalili, M.M. Hashemi, Tetrahedron Lett. 49 (2008) 2575.
L. Nagarapu, S. Apuri, S. Kentevari, J. Mol. Catal. 266 (2007) 104.
R. Hekmat Shoar, G. Rahimzadeh, F. Derikvand, M. Farzaneh, Synth. Commun. 40 (2010) 1270.
H. Shaterian, M. Ranjbar, J. Mol. Liq. 160 (2011) 40.
S.D. Sharma, P. Hazarika, D. Konwar, Tetrahedron Lett. 49 (2008) 2216.
R.S. Joshi, P.G. Mandhane, M.U. Shaikh, R.P. Kale, C.H. Gill, Chin. Chem. Lett. 21 (2010) 429.
M.G. Shen, C. Cia, W.B. Yi, J. Fluorine Chem. 129 (2008) 541.
A.R. Khosropour, Ultrason. Sonochem. 15 (2008) 659.
M.M. Heravi, K. Bakhtiari, H.A. Oskooie, S. Taheri, J. Mol. Catal. 263 (2007) 279.
M. Kidwai, P. Mothsra, V. Bansal, R.K. Somvanshi, A.S. Ethayathulla, S. Dey, T.P. Singh, J. Mol. Catal. 265 (2007) 177.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaterian, H.R., Ranjbar, M. & Azizi, K. Synthesis of highly substituted imidazoles using Brønsted acidic ionic liquid, triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate, as teusable catalyst. JICS 8, 1120–1134 (2011). https://doi.org/10.1007/BF03246570
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03246570