Abstract
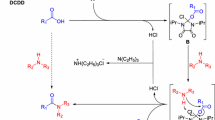
A quick and efficient, one-pot synthesis of carbazates was accomplished in high yields by the reaction of various primary, secondary, and tertiary alkyl halides with a variety of substituted hydrazines using Amberlite IRA 400 basic resin/CO2 system. The reaction conditions were mild with simpler work-up procedures than the previously reported methods.
Similar content being viewed by others
References
U. Ragnarsson, Chem. Soc. Rev. 30 (2001) 205
E.U. Schmidt, Hydrazine and its Derivatives Preparation, Properties, Applications, 2nd ed., Wiley- Interscience, New York, 2001.
M.S.C. Pedras, M. Jha, Bioorg. Med. Chem. 14 (2006) 4958
Shailendra, N. Bharti, F. Naqvi, A. Azam, Helv. Chim. Acta 85 (2002) 2713
N. Bharti, M.R. Maurya, F. Naqvi, A. Azam, Bioorg. Med. Chem. Lett. 10 (2000) 2243
N. Bharti, M.R. Mannar, N. Fehmida, A. Bhattacharya, S. Bhattacharya, A. Azam, Eur. J. Med. Chem. 35 (2000) 481.
S.K. Sengupta, O.P. Pandey, P.G. Rao, Sugarcane Pathology 1 (1999) 279
H.S. Chen, Z.H. Li, Y.H. Han, Z.W. Wang Chin. Chem. Lett. 10 (1999) 365
K. Chaturvedi, A.K. Jaiswal, K.N. Mishra, O.P. Pandey, S.K. Sengupta, ACH-Models in Chemistry 135 (1998) 93
G.C. Briggs, C.L. Cornell, D.J. Mansfield, R.M. Turner, PCT Int Appl. 1999, WO9923066, CAN:130:337920 (1999).
T.J. Connolly, A.J. Crittal, A.S. Ebrahim, G. Ji, Org. Process. Res. Dev. 4 (2000) 526
M.S. Gordan, J.G. Krause, M.A. Linneman-Mohr, R.R. Parchue, Synthesis 3 (1998) 244
M.J. Hauser, J. Org. Chem. 31 (1966) 968.
S. Sakakibara, I. Honda, M. Naruse, M. Kanaoka, Experimentia 25 (1969) 576
S. Sakakibara, I. Honda, K. Takada, M. Miyoshi, T. Ohnishi, K. Okumura Bull. Chem. Soc. Jpn. 42 (1969) 809
M. Quibell, W.G. Turnell, T. Johnson, J. Chem. Soc. Perkin. Trans. I 22 (1993) 2843
C.J. Gray, M. Quibell, N. Baggett, T. Hammerle, Int. J. Pept. Protien Res. 40 (1992) 351.
J.Y. Huang, H.S. Choi, D.H. Lee, S.E. Yoo, Y.D. Gong, J. Comb. Chem. 7 (2005) 136
J.Y. Hwang, H. S. Choi, D.H. Lee, Y.D. Gong, J. Comb. Chem. 7 (2005) 816.
C. Bolzati, E. Benini, M. Cavazza-Ceccato, E. Cozzala, E. Malago, S. Agostini, F. Tisato, F. Rofosco, G. Bandoli, Bioconjugate Chemistry 17 (2006) 419
M.A. Ali, A.H. Mirza, R.J. Butcher, K.A. Krause, Transit. Metal Chem. 31 (2006) 79
R. Singh, N.K. Kaushik, Main Group Met. Chem. 27 (2004) 327.
H. Dyker, J. Scherkenbeck, D. Gondol, A. Goehrt, A. Harder, J. Org. Chem. 66 (2001) 3760.
A.S. Dutta, J.S. Morley, J. Chem. Soc. Perkin Trans. I 1712 (1975)
S. Nara, T. Sakamoto, E. Miyazawa, Y. Kikugawa, Synth. Commun. 33 (2003) 87.
A. Saxena, J.P. Tandon, Polyhedron 2 (1983) 443
D.L. Fox, J.T. Ruxer, R.M. Liver, K.L. Alford, R.N. Salvatore, Tetrahedron Lett. 45 (2004) 401.
For Reviews see: a) D. Chaturvedi, S. Ray, Curr. Org. Chem. 11 (2007) 987
D. Chaturvedi, N. Mishra, V. Mishra, Curr. Org. Synthesis 3 (2007) 308; For our research work see
D. Chaturvedi, A. Kumar, S. Ray, Synth. Commun. 32 (2002) 2651
D. Chaturvedi, A. Kumar, S. Ray, Tetrahedron Lett. 44 (2003) 7637
D. Chaturvedi, S. Ray, Tetrahedron Lett. 47 (2006) 1307
D. Chaturvedi, S. Ray, Tetrahedron Lett. 48 (2007) 149
D. Chaturvedi, N. Mishra, V. Mishra, Tetrahedron Lett. 48 (2007) 5043
D. Chaturvedi, N. Mishra, V. Mishra, Monatsh. Chem. 139 (2008) 267
D. Chaturvedi, N. Mishra, V. Mishra, Synthesis (2008) 355
D. Chaturvedi, S. Ray, Monatsh. Chem. 137 (2006) 201
D. Chaturvedi, S. Ray, Monatsh. Chem. 137 (2006) 311
D. Chaturvedi, S. Ray, Monatsh. Chem. 137 (2006) 459
D. Chaturvedi, S. Ray, Monatsh. Chem. 137 (2006) 465
D. Chaturvedi, S. Ray, Monatsh. Chem. 137 (2006) 1219.
D. Chaturvedi, S. Ray, Lett. Org. Chem. 2 (2005) 742
D. Chaturvedi, S. Ray, J. Sulfur Chem. 26 (2005) 365
D. Chaturvedi, S. Ray, J. Sulfur Chem. 27 (2006) 265
D. Chaturvedi, N. Mishra, V. Mishra, J. Sulfur Chem. 28 (2007) 39
D. Chaturvedi, N. Mishra, V. Mishra, Chinese Chem. Lett. 17 (2006) 1309
D. Chaturvedi, N. Mishra, V. Mishra, J. Sulfur Chem. 28 (2007) 607.
D. Chaturvedi, A. Kumar, S. Ray, Ind. J. Chem. Sec. B 42B (2004) 437.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaturvedi, D., Chaturvedi, A.K., Mishra, N. et al. Basic resin mediated efficient one-pot synthesis of carbazates from the corresponding alkyl halides. JICS 6, 510–513 (2009). https://doi.org/10.1007/BF03246528
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03246528