Abstract
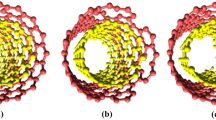
Adsorption of molecular hydrogen on single-walled carbon nanotube (SWCNT), sulfur-intercalated SWCNT (S-SWCNT), and boron-doped SWCNT (BSWCNT), have been studied by means of density functional theory (DFT). Two methods KMLYP and local density approximation (LDA) were used to calculate the binding energies. The most stable configuration of H2 on the surface of pristine SWCNT was found to be on the top of a hexagonal at a distance of 3.54 Å in good agreement with the value of 3.44 Å reported by Han and Lee (Carbon, 2004, 42, 2169). KMLYP binding energies for the most stable configurations in cases of pristine SWCNT, S-SWCNT, and BSWCNT were found to be −2.2 kJ mol−1, −3.5 kJ mol−1, and −3.5 kJ mol−1, respectively, while LDA binding energies were found to be −8.8 kJ mol−1, −9.7 kJ mol−1, and −4.1 kJ mol−1, respectively. Increasing the polarizability of hydrogen molecule due to the presence of sulfur in sulfur intercalated SWCNT caused changes in the character of its bonding to sulfur atom and affected the binding energy. In H2-BSWCNT system, stronger charge transfer caused stronger interaction between H2 and BSWCNT to result a higher binding energy relative to the binding energy for H2-SWCNT.
Similar content being viewed by others
References
V.V. Simonyan, P. Diep, J.K. Johnson, J. Chem. Phys. 1999, 111, 9778.
G.E. Froudakis, Rev. Adv. Mater. Sci. 2003, 5, 259.
V.V. Simonyan, J.K. Johnson, J. Alloys & Comp. 2002, 330, 659.
X. Chen, Y. Zhang, X.P. Gao, G.L. Pan, X.Y. Jiang, J.Q. Qu, F. Wu, J. Yan and D.Y. Song, Int. J. Hydrogen Energy, 2004, 29, 743 and references cited there.
E.L. Pace and A.R. Siebert J. Phys. Chem. 1995, 63, 1398.
G. Mpourmpakis, E. Tylianakis, D. Papanikolaou, and G. Froudakis, Rev. Adv. Mater. Sci. 2006, 11, 92.
S. Iijima, Nature. 1991, 354, 56.
L. Firlej, B. Kuchta, C. Wexler and P. Pfeifer, Adsorption, 2009, 15, 312.
S.H. Jhi and Y.K. Kwon, Phys. Rev. B 2004, 69, 245407.
V. Gayathri and R. Geetha, Adsorption, 2007, 13, 53.
R.T. Yang, Carbon, 2000, 38, 623. M.C. Nutzenadel, A. Nuttel, D. Chartuni and L. Schlapbach, Electrochem. Solid-State Lett. 1999, 2, 30.
X. Qin, X.P. Gao, H. Liu, H.T. Yuan, D.Y. Yan, W.L. Gong and D.Y. Song, Electrochem. Solid-State Lett. 2000, 3, 532.
K.F. Kelly, I.W. Chiang, E.T. Mickelson, R.H. Hauge, J.L. Margrave, X. Wang, G.E. Scuseria, C. Radloff and N.J. Halas, Chem. Phys. Lett. 1999, 313, 445.
A. Cao, H. Zhu, X. Zhang, X. Li, D. Ruan, C. Xu, B. Wei, J. Liang and D. Wu, Chem. Phys. Lett. 2001, 342, 510.
M. Volpe and F. Cleri, Chem. Phys. Lett. 2003, 371, 476.
R. Yang, Carbon, 2000, 38, 623.
A.M. Rao, P.C. Eklund, S. Bandow, A. Thess, R.E. Smalley, Nature, 1997, 388, 257.
R.S. Lee, H.J. Kim, J.E. Fischer, A. Thess, R.E. Smalley, Nature, 1997, 388, 255.
L. Grigorian, K.A. Williams, S. Fang, G.U. Sumanasekera, A.L. Loper, E.C. Dickey, S.J. Pennycook, P.C. Eklund, Phys. Rev. Lett. 1998, 80, 5560.
L. Grigorian, G.U. Sumanasekera, A.L. Loper, S. Fang, J.L. Allen, P. Eklund, Phys. Rev. B 1998, 58, R4195.
X. Fan, E.C. Dickey, P.C. Eklund, K.A. Williams, L. Grigorian, R. Buczko, S.T. Pantelides, S.J. Pennycook, Phys. Rev. Lett. 2000, 84, 4621.
I. Cabria, M.J. Lopez, J.A. Alonso, Eur. Phys. J. D 2005, 34, 279
E. Rangel, G. Ruiz-Chavarria, L.F. Magana, J.S. Arellano, Phys. Lett. A. 2009, 373, 2588.
E. Durgun, Y.R. Jang and S. Ciraci, Phys. Rev. B 2007, 76, 073413.
P. Chen, X. Wu, J. Lin and K.L. Tan, Science, 1999, 285, 91.
K.F. Kelly, I.W. Chiang, E.T. Mickelson, R.H. Hauge, J.L. Margrave, X. Wang, G.E. Scuseria, C. Radloff and N.J. Halas, Chem. Phys. Lett. 1999, 313, 445.
A. Cao, H. Zhu, X. Zhang, X. Li, D. Ruan, C. Xu, B. Wei, J. Liang and D. Wu, Chem. Phys. Lett. 2001, 342, 510.
R.G. Ding, G.Q. Lu, Z.F. Yan and M.A. Wilson, J. Nanosci. Nanotech. 2001, 1, 7.
S.H. Jhi and Y.K. Kwon, Phys. Rev. B. 2004, 69, 245407.
R. Wang, D. Zhang, Y. Zhang and Ch. Liu, J. Phys. Chem. B. 2006, 110, 18267.
Z. Zhou, X. Gao, J. Yan, D. Song, Carbon, 2006, 44, 939.
M. Sankaran and B. Viswanathan, Carbon, 2006, 44, 2816.
Y. Zhao; Y.H. Kim; A.C. Dillon; M.J. Heben and S.B. Zhang, Phys. Rev. Lett. 2005, 94, 155504.
G. Guo, F. Wang, H. Sun, D. Zhang, Int. J. Quantum Chem. 2008, 108, 203.
M.J. Frisch et al. Gaussian 03, Revision B.01,Gaussian, Inc., Pittsburgh PA, 2003
M.J.S. Dewar and C.H. Reynolds, J. Comp. Chem. 1988, 2, 140.
J.K. Kang and C.B. Musgrave, J. Phys. Chem. 2001, 115, 11040.
P. Hohenberg and W. Kohn, Phys. Rev. B. 1964, 136, 864.
C. Lee, W. Yang and R.G. Parr. Phys. Rev. B 1988, 37, 785.
S.H. Vosko, L. Wilk, M. Nusair, Canadian J. Phys. 1980, 58, 1200.
D.M. Ceperley and B.J. Alder, Phys. Rev. Lett. 1980, 45, 566.
C.D. Sherrill, M.S. Lee, and M. Head-Gordon, Chem. Phys. Lett. 302, 425, 1999.
S.S. Han and H.M. Lee, Carbon, 2004, 42, 2169.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mousavipour, S.H., Chitsazi, R. A theoretical study on the effect of intercalating sulfur atom and doping boron atom on the adsorption of hydrogen molecule on (10,0) single-walled carbon nanotubes. JICS 7 (Suppl 2), S92–S102 (2010). https://doi.org/10.1007/BF03246188
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03246188