Abstract
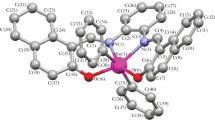
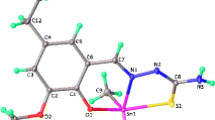
The new organotin(IV) complexes with 4-({[(E)-(2-hydroxyphenyl)methylidene]amino}methyl)cyclohexane carboxylic acid (HL, Schiff base) were synthesized by the reaction of di- and triorganotin salts in the presence of triethylamine as base or dioctyltin oxide using Dean and Stark trap for the removal of azeotropic water. All complexes were characterized by elemental analysis, IR, NMR (1H and 13C) and mass spectrometry. The IR data indicate that in both di- and triorganotin(IV) carboxylates, the ligand moiety -COO acts as a bidentate group in solid state. Multinuclear NMR data show that triorganotin complexes exhibit the four-coordinated geometry, while diorganotin(IV) complexes show the coordination number greater than four, probably five or six, in solution state. These compounds were screened for antibacterial activities against six pathogenic bacterial strains. The activities were measured in terms of inhibition zones (mm). Antifungal activity was determined against six pathogenic fungal strains, cytotoxicity by the brine shrimp lethality assay. Results for antibacterial and antifungal activity, and cytotoxicity of these compounds demonstrate that complexes exhibit significant biological activity with few exceptions.
Similar content being viewed by others
References
L. Pellerito, L. Nagy, Coord. Chem. Rev. 224 (2002) 111.
E.R.T. Tiekink, Appl. Organomet. Chem. 1 (1991) 5.
C.J. Evans, S. Karpel, J. Organomet. Chem. 1 (1985) 16.
M.F. Mahon, K.C. Molloy, P.C. Waterfield, J. Organomet. Chem. 361 (1989) C5.
P. Quevauviller, R. Ritsema, R. Morabit, W.M.R. Dirkx, Appl. Organomet. Chem. 541 (1994) 8.
S. Belwal, R.K. Saini, R.V. Singh, Indian J. Chem. 37A (1998) 245.
A.J. Crowe, Appl. Organomet. Chem. 1 (1987) 143.
E.R.T. Tiekink, Appl. Organomet. Chem. 1 (1991) 5.
I. Sakiyan, E. Logoglu, S. Arslan, N. Sari, N. Sakiyan, Bio-Met. 17 (2004) 115.
Z.H. Chohan, M. Hassan, K.M. Khan, C.T. Supuran, J. Enz. Inhib. Med. Chem. 20 (2005) 183.
M. Danish, S. Choudhary, M.R. Churchill, K.M. Keil, A.V. Eliseev, J.R. Morrow, Inorg. Chem. 40 (2001) 1591.
R. Karmakar, C.R. Choudhury, S. Mitra, L. Dahlenburg, Struct. Chem. 16 (2005).
S.K. Sahu, V. Chakravortty. J. Radioanal. Nucl. Chem. 227 (1998) 163.
S. Shahzadi, K. Shahid, S. Ali, Russian. J. Coord. Chem. 33 (2007) 403.
K. Shahid, S. Shahzadi, S. Ali, M. Mazhar, Bull. Korean Chem. Soc. 27 (2006) 44.
W.L.F. Armarego, C.L.L. Chai, Purification of Laboratory Chemicals, 5th ed., Butterworth-Heinemann, London, 2003.
H. Unver, Spectrosc. Lett. 34 (2001) 783.
M. Parvez, S. Ahmad, S. Ali, M.H. Bhatti, M. Mazhar, Acta Cryst. E60 (2004) m554.
L.Q. Xie, Z.Q. Yang, Z.X. Zhang, D.K. Zhang, Appl. Organomet. Chem. 6 (1992) 193.
Q. Xie, Z. Yang, L. Jiang, Main Group Met. Chem. 19 (1996) 509.
H.D. Yin, C.H. Wang, Y. Wang, C.L. Ma, J.X. Shao, J.H. Zhang, Acta Chim. Sinica 60 (2002) 143.
M. Danish, S. Ali, A. Badshah, M. Mazhar, H. Masood, A. Malik, G. Kehr, Synth. React. Inorg. Met.-Org. Chem. 27 (1997) 863.
H.O. Kalinowski, S. Berger, S. Braun, Carbon NMR Spectroscopy, Chichester, John Wiley & Sons, UK, 1988.
M. Nadvornik, J. Holecek, K. Handlir, A. Lycka, J. Organomet. Chem. 275 (1984) 43.
J. Holeček, A. Lycka, Inorg. Chim. Acta 118 (1986) L15.
M. Danish, S. Ali, M. Mazhar, A. Badshah, M.I. Choudhary, H.G. Alt, G. Kehr, Polyhedron, 14 (1995) 3115.
M. Gielen, M. Boualam, E.R.T. Tiekink, Appl. Organomet. Chem. 8 (1994) 19.
A. Rahman, M.I. Choudhary, W.J. Thomsen, Bioassay Techniques for Drug Development, Hardward Academic Press, Amsterdam, 2001.
M. Jain, S. Gaur, V.P. Singh, R.V. Singh, Appl. Organomet. Chem. 18 (2004) 73.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, F.A., Ali, S. & Shahzadi, S. Spectral and biological studies of newly synthesized organotin(IV) complexes of 4-({[(E)-(2-hydroxyphenyl)methylidene]amino}methyl)cyclohexane carboxylic acid Schiff base. JICS 7, 59–68 (2010). https://doi.org/10.1007/BF03245860
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03245860