Abstract
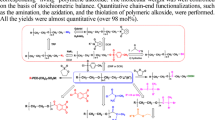
Then-butyllithium-initiated ring-opening polymerization of ethylene oxide, in a mixture of benzene and dimethylsulfoxide (DMSO), between 25-45°C, with potassiumtert-butoxide, is a useful and powerful method to control the molecular weight as well as achieve a quantitative chain-end functionalization yield of the resulting polymeric alkoxidevia a one pot synthesis. The molecular weight of the product could be controlled by adjusting the ratio of grams of monomer to moles of initiators, such asn-butyllithium ([n-BuLi]) and potassiumt-butoxide ([t-BuOK]). The yields for the macromonomer and?-brominated poly(ethylene oxide) (PEO) were quantitative in relation to the chain-end functionalizations of the polymeric alkoxide formed. The resulting products were characterized by a combination of1H-NMR spectroscopic and size exclusion chromatographic analyses.
Similar content being viewed by others
References
Advances in lithium-ion batteries, B. Scrosati and W. Schalkwijk, Eds., Plenum, New York, 2002.
J.-M. Tarascon and M. Armand,Nature,414, 359 (2001).
F. Croce, R. Curini, A. Martinelli, L. Persi, F. Ronci, B. Scrosati, and R. Caminiti,J. Phys. Chem. B,103, 10632 (1999).
D. Swierczynski, A. Zalewska, and W. Wieczorek,Chem. Mater.,13, 1560 (2001).
H.-M. Xiong, D.-P. Liu, H. Zhang, and J.-S. Chen,J. Mater. Chem.,14, 2775 (2004).
J. M. Harris and R. B. Chess,Nature Rev. Drug Disc.,2, 214 (2003).
R. Duncan,Nature Rev. Drug Disc.,2, 347 (2003).
M. Sharpe, S. E. Easthope, G. M. Keating, and H. M. Lamb,Drugs,62, 2089 (2002).
R. Satchi-Tainaro, R. Duncan, and C. M. Barnes,Adv. Polym. Sci.,193, 1 (2006).
J.-F. Lutz and A. Hoth,Macromolecules,39, 893 (2006). (a) T.-L. Cheng, C.-M. Cheng, B.-M. Chen, S.-A. Tsao, K.-H. Chuang, S.-W. Hsiao, Y.-H. Lin, and S. R. Roffler,Biconjugate Chem.,16, 1225 (2005); (b) W. S. Shim, J. S. Lee, and D. S. Lee,Macromol. Res.,13, 344 (2005).
R. P. Quirk,Anionic synthesis of polymers with functional groups, inComprehensive Polymer Science, S. L. Aggarwal and S. Russo, Eds., Pregamon Press, Seoul, 1992, Suppl.
R. P. Quirk and J. Kim,Macromonmers and Macroinitiators, inRing-opening Polymerization; Mechanisms, Catalysis, Structure, Utility, D. J. Brunelle, Ed., Hanser, New York, 1993, Chap. 9, pp 263–293.
S. Slomkowski and A. Duda,Anionic Ring-Opening Polymerization, inRing-opening Polymerization; Mechanisms, Catalysis, Structure, Utility, D. J. Brunelle, Ed., Hanser, New York, 1993, Chap. 3, pp 87–128.
H. L. Hsieh and R. P. Quirk,Anionic Polymerization: Principles and Practical Applications, Marcel Dekker, New York, 1996, Chap. 24, pp 685–710.
D. H. Richards and M. Szwarc,Trans. Faraday Soc.,55, 1644 (1959);
L. E. St. Pierre and C. C. Price,J. Am. Chem. Soc.,78, 3432 (1956).
B. Esswein and M. Moller,Angew. Chem. Int. Ed. Engl.,35, 623 (1996).
R. P. Quirk, J. Kim, C. Kausch, and M. Chun,Polym. Int.,39, 3 (1996).
R. P. Quirk, J. Kim, K. Rodrigues, and W. L. Mattice,Makromol. Chem., Macromol. Symp.,42/43, 463 (1991).
M. Morton and L. J. Fetters,Rubber Chem. Technol.,48, 359 (1975).
J. M. Stouffer and T. J. McCarthy,Macromolecules,21, 1204 (1988).
H. Gilman and F. K. Cartledge,J. Organomet. Chem.,2, 447 (1964).
A. Napoli, N. Tirelli, G. Kilcher, and J. A. Hubbell,Macromolecules,34, 8913 (2001).
R. P. Quirk and J. J. Ma,J. Polym. Sci.; Part A: Polym. Chem.,24, 2031 (1988).
V. Halaska, L. Lochmann, and D. Lim,Collect. Czec. Chem. Commun.,33, 3245 (1968).
J. E. Figueruelo and D. J. Worsfold,Eur. Polym. J.,4, 439 (1968).
M. Schlosser,J. Organomet. Chem.,8, 9 (1967).
L. Brandsma and H. Verkruijsse,Preparative Polar Organometallic Chemistry 1, Springer-Verlag, Berlin Heidelberg, 1987, Chap. II, p 41.
C. E. H. Bawn, A. Ledwith, and N. McFarlane,Polymer,10, 653 (1969).
C. C. Price and D. D. Carmelite,J. Am. Chem. Soc.,88, 4039 (1966).
H. L. Hsieh and R. P. Quirk,Anionic Polymerization: Principles and Practical Applications, Marcel Dekker, New York, 1996, Chap. 4, pp 71–92.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J., Choi, Sy., Kim, K.M. et al. An efficient method for synthesis of PEO-based macromonomer and macroinitiator. Macromol. Res. 15, 337–342 (2007). https://doi.org/10.1007/BF03218796
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF03218796