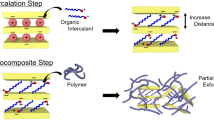
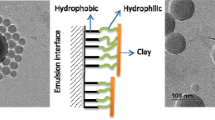
Abstract
The approach studied in the present work produced an exfoliated state of clay layers via confinement of the charged nano-sized polystyrene (PS) beads within the gallery of swollen pristine clay. It was demonstrated that adsorption of the polymer nanobeads dramatically promotes expansion of the clay gallery. A comparative study of incorporation was conducted by employing organo-modified clay along with two different colloid polymer systems: electrostatically stabilized PS nanobeads and cationic monomer-grafted PS nanobeads. The mechanism of adsorption of the monomer-grafted polymer beads onto clay via cationic exchange between the alkyl ammonium group of the polymer nanobeads and the interlayer sodium cation of the layered silicate was verified by using several techniques. As distinct from the polymer nanobeads formed using conventional miniemulsion polymerization method, competitive adsorption of stabilizing surfactant molecules was be prevented by grafting the surface functional groups into the polymer chain, thereby supporting the observed effective adsorption of the polymer beads. The presence of surface functional groups that support the establishment of strong polymer-clay interactions was suggested to improve the compatibility of the clay with the polymer matrix and eventually play a crucial role in the performance of the final nanocomposites.
Similar content being viewed by others
References
R. Y. Lochhead, C. M. Boykin, and C. McConnell, inPolymer Nanocomposites, R. A. Vaia and R. Krishnamoorti, Eds., Oxford University Press, Cary, NC, 2002, p. 43.
M. Alexandre and P. Dubois,Mater. Sci. Eng. R: Reports,28, 1 (2000).
Z. Wang, J. Massam, and T. J. Pinnavaia, inPolymer-Clay Nanocomposites, T. J. Pinnavaia and G. W. Beall, Eds., John Wiley & Sons, Chichester, 2000, p. 127.
G. Lagaly and T. J. Pinnavaia,Appl. Clay Sci.,15, 1 (1999).
R. A. Vaia, inPolymer-Clay Nanocomposites, T. J. Pinnavaia, and G. W. Beall, Eds., John Wiley & Sons, Chichester, 2000, p. 229.
C. I. Park, O. O. Park, J. G. Lim, and H. J. Kim,Polymer,42, 7465 (2001).
R. A. Vaia and E. P. Giannelis,Macromolecules,30, 8000 (1997).
R. A. Vaia, K. D. Jandt, E. J. Kramer, and E. P. Giannelis,Chem. Mater.,8, 2628 (1996).
P. C. LeBaron, Z. Wang, and T. J. Pinnavaia,Appl. Clay Sci.,15, 11 (1999).
Y. Li and H. Ishida,Polymer,44, 6571 (2003).
N. Ogata, S. Kawakage, and T. Ogihara,J. Appl. Polym. Sci.,66, 573 (1997).
J. Billingham, C. Breen, and J. Yarwood,Vib. Spectrosc.,14, 19 (1997).
R. L. Parfitt and D. J. Greenland,Clay Miner.,8, 305 (1970).
E. A. Ruiz-Hitzky, Casal Pilar, Galvan Blanca, and C. Juan,Adv. Mater.,7, 180 (1995).
X. Zhao, K. Urano, and S. Ogasawara,Colloid Polym. Sci.,267, 899 (1989).
M. Kato and A. Uzuki, inPolymer Clay Nanocomposites, R. A. Vaia and R. Krishnamoorti, Eds., John Wiley & Sons, Chichester, 2000, p. 97.
X. Fu and S. Qutubuddin,Mater. Lett.,42, 12 (2000).
S. S. Hou and K. Schmidt-Rohr,Chem. Mater.,15, 1938 (2003).
H. M. Jeong and Y. T. Ahn,Macromol. Res.,13, 102 (2005).
H. M. Jeong, M. Y. Choi, and Y. T. Ahn,Macromol. Res.,14, 312 (2006).
X. Huang and W. J. Brittain,Macromolecules,34, 3255 (2001).
G. B. Rossi, G. Beaucage, T. D. Dang, and R. A. Vaia,Nano Lett.,2, 319 (2002).
R. G. Gilbert,Emulsion Polymerization. A Mechanistic Approach, Academic Press, London, 1995.
Z. Liu, H. Xiao, N. Wiseman, and A. Zheng,Colloid Polym. Sci.,281, 815 (2003).
H. van Olphen,An Introduction to Clay Colloid Chemistry, Interscience Publications, New York, 1963.
R. M. Fitch,Polymer Colloids: A Comprehensive Introduction, Academic Press San Diego, 1997.
S. S. Madaeni and M. Ghanbarian,Polym. Int.,49, 1356 (2000).
G. Beamson and D. Briggs,High Resolution Xps of Organic Polymers: The Scienta Esca300 Database, Wiley & Sons Ltd., Chichester, 1992.
J. M. Adams, S. Evans, P. I. Reld, J. M. Thomas, and M. J. Walters,Anal. Chem.,49, 2001 (1977).
N. C. Dutta, T. Iwasaki, T. Ebina, and H. Hayashi,J. Colloid Interf. Sci.,216, 161 (1999).
H. van Olphen,Clay Colloid Chemistry for Clay Technologists, Geologists, and Soil Scientists, John Wiley & Sons, New York, 1963.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khvan, S., Kim, J. & Lee, SS. Fabrication of pre-exfoliated clay masterbatch via exfoliation-adsorption of polystyrene nanobeads. Macromol. Res. 15, 51–58 (2007). https://doi.org/10.1007/BF03218752
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF03218752