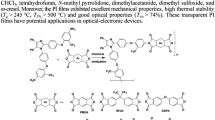
Abstract
We have synthesized a water-soluble polyimide precursor, poly(amic acid) amine salt (PAD), from pyromellitic dianhydride (PMDA), 4,4′-oxydianiline, andN,N′-dimethylethanolamine (DMEA) and have investigated in detail its properties with respect to the degree of salt formation (D sf ). The maximum value ofD sf we obtained upon precipitation of the precursor solution into acetone was 79%. We synthesized a PAD having aD sf of 100% (PAD100) by the solid state drying of an organic solution. The precursors showed different solubility depending on theD sf to make up to 4 wt% solutions in water containing a small amount of DMEA. PAD100 is completely soluble in pure water. We investigated the imidization behavior of PAD in aqueous solution using various spectroscopic methods, which revealed that PAD100 has faster imidization kinetics relative to that of the poly(amic acid)-type precursors. The resulting polyimide films prepared from an aqueous precursor solution possess almost similar physical and thermal properties as those prepared from N-methyl-2-pyrrolidone(NMP) solution. Therefore, we have demonstrated that PAD can be used as a water-based precursor of polyimide; this procedure avoids the use of toxic organic solvents, such as NMP.
Similar content being viewed by others
References
N. Yoda and H, Hiramoto,J. Macromol. Sci. Chem.,A21, 1641 (1984).
S. Kubota, T. Moriwaki, T. Ando, and A. Fukami,J. Appl. Polym. Sci.,33, 1763 (1987).
Q. Li, T. Yamashita, K. Horie, H. Yoshimoto, T. Miwa, Y. Maekawa,J. Polym. Sc., Part A: Polym. Chem.,36, 1329 (1998).
Y. Ding, B. Bikson, and J. K. Nelson,Macromolecules,35, 905 (2002).
J. A. Kreuz, A. L. Endrey, F. P. Gay, and C. E. Sroog,J. Polym. Sci., Part A: Polym. Chem.,4, 2607 (1966).
R. J. W. Reynolds and J. D. Seddon,J. Polym. Sci., Part C: Polym. Lett.,23, 45 (1968).
C. E. Sroog, A. L. Endrey, S. V. Abramo, C. E. Berr, W. M. Edward, and K. L. Olivier,J. Polym. Sci., Part A: Polym. Chem.,3, 1373 (1965).
A. L. Endrey,U. S. Pat. 3,179,631 (1965).
I. Kardash, A. Y. Ardashnikov, F. S. Yakushin, and A. N. Pravednikov,Polym. Sci. USSR,17, 689 (1975).
N. C. Stoffel, E. J. Krame, W. Volksen, and T. P. Russell,Polymer,34, 4524 (1993).
J. V. Facinelli, S. L. Gardner, L. Dong, C. L. Sensenich, R. M. Davis, and J. S. Riffle,Macromolecules,29, 7342 (1996).
K. H. Yu, Y. H. Yoo, J. M. Rhee, M.-H. Lee, and S.-C. Yu,Mat. Res. Innov.,7, 51 (2003).
H. Han, C. C. Gryte, and M. Ree,Polymer,36, 1663 (1995).
C. A. Pryde,J. Polym. Sci., Part A: Polym. Chem.,27, 711 (1989).
S. Gan,J. Sci. Eng. Comp. Mater.,2, 119 (1992).
K. Kim, S. Choi, S. Kim, S. Chao, and C. Wang,J. Mat. Sci.,28, 1537 (1993)
T. Nishino, M. Kotera, N. Inayoshi, N. Miki, and K. Nakamae,Polymer,41, 6913 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yang, J., Lee, MH. A water-soluble polyimide precursor: Synthesis and characterization of poly(amic acid) salt. Macromol. Res. 12, 263–268 (2004). https://doi.org/10.1007/BF03218398
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF03218398