Abstract
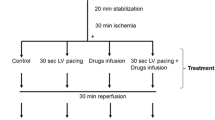
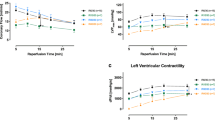
Deceasing sarcoplasmic reticular (SR) calcium may contribute to the myocardiac protection against ischemia and reperfusion-induced injury. Therefore, using the isolated working rat heart model, we investigated the effect of Thapsigargin (TH)-induced SR calcium diminution on the myocardial protection when added either before onset of ischemia or at time of reperfusion under conditions of normothermic ischemia. Hearts (n=6/group) from male Wistar rats were aerobically (37°C) perfused (20 min) with bicarbonate buffer. In the experimental protocol A, this was followed by a 3 min infusion of St. Thomas’ Hospital cardioplegic solution No. 2 (STS) containing various concentrations of TH. Hearts were then subjected to 34 min of normothermic (37°C) global ischemia and 35 min of reperfusion (15 min Langendorff, 20 min working). Reperfusion cardiac functions at 20 min of working perfusion was measured and compared with the preischemia values. STS added to 0.1 and 0.25 μmol/L TH improved recovery of aortic flow after 20 min reperfusion from 47 ± 3% in the TH free controls to 62 ± 3, 63 ± 2% (n=6) (p<0.05). There was no difference in creatine kinase (CK) leakage during Langendorff reperfusion between the TH treated groups and the control group. In the experimental protocol B, 3 min of cardioplegia without TH and 34 min of ischemia (37°C) were followed by a 10 min Langendorff reperfusion with various concentrations of TH, then 10 min Langendroff reperfusion for washing out, and 20 min working reperfusion. When TH was added to reperfusate the recovery of aortic flow did not change. 0.5 μmol/L TH group had the detelious effect. Thus, TH, when added to the cardioplegia, enhanced myocardial protection. We conclude that lessened uptake of Ca2+ into sarcoplasmic reticulum by inhibitors of the Ca2+-ATPase pump can decrease ischemia and reperfusion-induced injury.
Similar content being viewed by others
References
Chien KR, Han A, Sen A, Buja LM, Willerson JT: Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation-reacylation cycle and the depletion of membrane phospholipids. Circ Res 54: 313–322, 1984
Steenbergen C, Hill ML, Jennings RB: Cytoskeletal damage during myocardial ischemia. Changes in vinculin immunofluorescence staning during total in vitro ischemia in canine heart. Circ Res 60: 478–486, 1987
Kawaguchi H, Yasuda H: Prostacycline biosynthesis and phospholipase activity in hypoxic rat myocardium. Circ Res 62: 1175–1181, 1988
Tani M, Neely JR: Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involment of H+-Na+ and Na+-Ca2+ exchange. Circ Res 65: 1045–1056, 1989
Grinwald PM, Brosnahan C: Sodium imbalance as a cause of calcuim overload in post-hypoxic reoxygenation injury. J Mol Cell Cardiol 19: 487–495, 1987
Yamamoto F, Manning AS, Braimbridge MV, Hearse DJ: Cardioplegia and slow channel blockers. Studies with verapamil. J Throac Cardiovasc Surg 86: 252–261, 1983
Yamamoto F, Manning AS, Braimbridge MV, Hearse DJ: Calcium antagonists and myocardial protection. Diltiazem during cardioplegic arrest. Throac Cadiovasc Surgeon 31: 369–373, 1983
Feher JJ, LeBolt WR, Manson NH: Differential effect of global ischemia on the ryanodine-sensitive and ryanodine-insensitive calcium uptake of cardiac sarcoplasmic reticulum. Circ Res 65: 1400–1408, 1989
Davis MD, LeBolt WR, Feher JJ: Reversibilitiy of the effects of normothermic global ischemia on the ryanodine-sensitive and ryanodine-insensitive calcium uptake of cardiac sarcoplasmic reticulum. Circ Res 70: 163–171, 1992
Yamamoto H, Yamamoto F, lchikawa H, Takahashi A, Fujita T: Effects of ryanodine on myocardial ischemia and reperfusion induced injury in isolated rat heart [Abstract]. J Mol Cell Cardiol 22: S-58, 1990
Akita T, Abe T, Kato S, Kodama I, Toyama J: Protective effects of diltiazem and ryanodine against ischemia-reperfusion injury in neonatal rabbit heart. J Throac Cardiovasc Surg 106: 55–66, 1993
Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP: Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibiton of the endoplasmic reticulum Ca2+ ATPase. Proc Natl Acad Sci U.S.A. 87: 2466–2470, 1990
Moore GA, McConkey DJ, Kass GEN, O’Brien PJ, Orrenis S: 2,5-Di (tert-butyl)-l, 4-benzohydroquinone—a novel inhibitor of liver microsomal Ca2+ sequestration. FEBS Lett 224: 331–336, 1987
Seider NW, Jona I, Vegh M, Martonosi A: Cyclopiazonic acid is a specific inhibitior of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem 264: 17816–17823, 1989
Neely JR, Leiebermeister H, Battersby EJ, Morgan HE: Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol 212: 804–814, 1967
Hearse DJ, Braimbridge MV, Jynge P: Model and markers for the invastigation of myocardial tissure damage., In: Hearse DJ, Braimbridge MV, Jynge P, eds. Protection of the Ischemic Myocardium. Cardioplegia. New York, 1981, Raven Press p50–92
du Toit EF, Opie LH: Inhibitors of Ca2+ ATPase pump of sarcoplasmic reticulum attenuate reperfusion stunning in isolated rat heart. J Cardiovasc Pharmacol 24: 678–684, 1994
Iuni M, Wang S, Saito A, Fleischer S: Characterization of junctional and longitudinal sarcoplasmic reticulum from heart muscle. J Biol Chem 263: 10843–10850, 1988
Kirby, Sagara, Gaa S, Inesi G, Lederer WJ, Rogers TB: Thapsigargin inhibits contraction and Ca2+ transient in cardiac cells by specific inhibition of the sarcoplasmic reticulum Ca2+ pump. J Biol Chem 267: 12545–12551, 1992
Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drøbak BK, Bjerrum PJ, Christensen SB, Hanley MR: Thapsigargin, novel molecular probe for studying intracellular calcium release and storage. Agents Actions 27: 17–23, 1989
Ohuchi K, Sugawara T, Watanabe M, Hirasawa N, Tsurufuji S, Fuhiki H, Chritensen SB, Sugiura T: Analysis of the stimulative effect of thapsigargin, a non-TPA-type tumor promoter, on arachidonic acid metabolism in rat peritoneal macrophages. Br J Pharmacol 94: 917–923, 1988
Takemura H, Thastrup O, Putney JW Jr: Calcium efflux across the plasma membrane of rat parotid acinar cells is unaffected by receotor activation or by the microsomal calcium ATPase inhibitor, thapsigargin. Cell Calcium 11: 11–17, 1990
Nakamura H, Nakasaki Y, Matsuda N, Shigekawa M: Inhibition of sarcoplasmic reticulum Ca2+-ATPase by 2,5-di(tert-butyl)-1.4-benzohydroquinone. J Biochem 112: 750–755, 1992
Takemura H, Hughes AR, Thrastrup O, Putney JW Jr: Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intraellular calcium pool, and not an inositol phosphate, regulate calcium fluxes at the plasma membrane. J Biol Chem 264: 12266–12271, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kumada, Y., Yamamoto, F., Yamamoto, H. et al. Decreasing sarcoplasmic reticular calcium gives rise to myocardial protection? —The effect of thapsigargin for myocardial protection under conditions of normothermia—. Jpn J Thorac Caridovasc Surg 46, 368–374 (1998). https://doi.org/10.1007/BF03217757
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03217757