Abstract
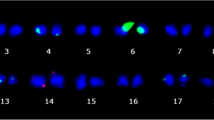
This study focuses on the variability of chromosomal location and number of ribosomal DNA (rDNA) sites in some diploid and autotetraploidFestuca pratensis andLolium perenne cultivars, as well as on identification of rDNA-bearing chromosomes in their triploid and tetraploidF. pratensis ×L. perenne hybrids. The rDNA loci were mapped using fluorescence in situ hybridization (FISH) with 5S and 25S rDNA probes, and the origin of parental genomes was verified by genomic in situ hybridization (GISH) withL. perenne genomicDNAas a probe, andF. pratensis genomic DNA as a block. FISH detected variation in the number and chromosomal location of both 5S and 45S rDNA sites. InF. pratensis mostly additional signals of 5S rDNA loci occurred, as compared with standardF. pratensis karyotypes. Losses of 45S rDNA loci were more frequent inL. perenne cultivars and intergeneric hybrids. Comparison of theF. pratensis andL. perenne genomes approved a higher number of rDNA sites as well as variation in chromosomal rDNA location inL. perenne. A greater instability ofF. pratensis-genome-like andL. perenne-genome-like chromosomes in tetraploid hybrids was revealed, indicating gains and losses of rDNA loci, respectively. Our data indicate that the rDNA loci physically mapped on chromosomes 2 and 3 inF. pratensis and on chromosome 3 inL. perenne are useful markers for these chromosomes in intergenericFestuca ×Lolium hybrids.
Similar content being viewed by others
References
Badaeva ED, Friebe B, Gill BS, 1996. Genome differentiation inAegilops. 2. Physical mapping of 5S and 18S–26S ribosomal RNA gene families in diploid species. Genome 39: 1150–1158.
Bennett ST, Kenton AY, Bennett MD, 1992. Genomicin situ hybridization reveals the allopolyploid nature ofMilium montianum (Gramineae). Chromosoma 101: 420–424.
Brysting AK, Holst-Jensen A, Leitch I, 2000. Genomic origin and organization of the hybridPoa jemtlandica (Poaceae) verified by genomicin situ hybridization and chloroplast DNA sequences. Ann Bot 85: 439–445.
Canter PH, Pašakinskiené I, Jones RN, Humphreys MW, 1999. Chromosome substitutions and recombination in the amphiploidLolium perenne ×Festuca pratensis cv. Prior (2n=4x=28). Theor Appl Genet 98: 809–814.
Chung MC, Lee YI, Cheng YY, Chou YJ, Lu CF, 2008. Chromosomal polymorphism of ribosomal genes in the genusOryza. Theor Appl Genet 116: 745–753.
Cuadrado A, Jouve N, 1997. Distribution of highly repeated DNA sequences in species of the genusSecale. Genome 40: 309–317.
Cuadrado A, Jouve N, 2002. Evolutionary trends of different repetitive DNA sequences during speciation in the genusSecale. J Heredity 93: 339–345.
Datson PM, Murray BG, 2006. Ribosomal DNA locus evolution inNemesia: transposition rather than structural rearrangement as the key mechanism? Chrom Res 14: 845–857.
Dubcovsky J, Dvorak J, 1995. Ribosomal RNA multigene loci: nomads of theTriticeae genomes. Genetics 140: 1367–1377.
Dydak M, Kolano B, Nowak T, Siwinska D, Maluszynska J, 2009. Cytogenetic studies of three European species ofCentaurea L. (Asteraceae). Hereditas 146(4): 152–161.
Frello S, Heslop-Harrison JS, 2000. Chromosomal variation inCrocus vernus Hill (Iridaceae) investigated byin situ hybridization of rDNA and a tandemly repeated sequence. Ann Bot 86: 317–322.
Gerlach WL, Dyer TA, 1980. Sequence organization of the repeating units in the nucleus of wheat which contain 5S rRNA genes. Nucl Acids Res 11: 4851–4865.
Harper JA, Thomas ID, Lovatt JA, Thomas HM, 2004. Physical mapping of rDNA sites in possible diploid progenitors of polyploid Festuca species. Plant Syst Evol 245: 163–168.
Hasterok R, Jenkins G, Langdon T, Jones RN, Maluszynska J, 2001. Ribosomal DNA is an effective marker ofBrassica chromosomes. Theor Appl Genet 103: 486–490.
Hasterok R, Wolny E, Hosiawa M, Kowalczyk M, Kulak-Ksiazczyk S, Ksiazczyk T, et al. 2006. Comparative analysis of rDNA distribution in chromosomes of various species of Brassicaceae. Ann Bot 97: 205–216.
Hayasaki M, Morikawa T, Tarumoto I, 2000. Intergenomic translocations of polyploid oats (genusAvena) revealed by genomicin situ hybridization. Genes Genet Syst 75: 167–171.
Hayasaki M, Morikawa T, Legget JM, 2001. Intraspecific variation of 18S-5.8S-26S rDNA sites revealed by FISH and RFLP in wild oat,Avena agadiriana. Genes Genet Syst 76: 9–14.
He S, Huang M, Huang J, Li J, Hu Y, Zhang L, et al. 2009. Dynamics of the evolution of the genus ofAgrostis revealed by GISH/FISH. Crop Sci 49: 2285–2290.
Huang J, Ma L, Yang F, Fei SZ, Li L, 2008. 45S rDNA regions are chromosome fragile sites expressed as gapsin vitro on metaphase chromosomes of root-tip meristematic cells inLolium spp. PLoS ONE 3(5): e2167. DOI:10.1371/journal.pone.0002167.
Jauhar PP, 1975. Chromosome relationships betweenLolium andFestuca (Gramineae). Chromosoma 52: 103–121.
Jiang J, Gill BS, 1994. New 18S.26S ribosomal RNA gene loci: chromosomal landmarks for the evolution of polyploid wheats. Chromosoma 103: 179–185.
Jones ES, Mahoney NL, Hayward MD, Armstead IP, Jones JG, Humphreys MO et al. 2002. An enhanced molecular marker based genetic map of perennial ryegrass (Lolium perenne) reveals comparative relationships with other Poaceae genomes. Genome 45: 282–295.
King IP, Morgan WG, Armstead IP, Harper JA, Hayward MD, Bollard A, et al. 1998. Introgression mapping in the grasses. I. Introgression ofFestuca pratensis chromosomes and chromosome segments intoLolium perenne. Heredity 81: 462–467.
King IP, Morgan WG, Harper JA, Thomas HM, 1999. Introgression mapping in the grasses. II. Meiotic analyses of theLolium perenne/Festuca pratensis triploid hybrids. Heredity 82: 107–112.
Kopecký D, Loureiro J, Zwierzykowski Z, Ghesquière M, Doležel J, 2006. Genome constitution and evolution inLolium ×Festuca hybrid cultivars (Festulolium). Theor Appl Genet 113: 731–742.
Kosmala A, Zwierzykowski Z, Gąsior D, Rapacz M, Zwierzykowska E, Humphreys MW, 2006. GISH/FISH mapping of genes for freezing tolerance transferred fromFestuca pratensis toLolium multiflorum. Heredity 96: 243–251.
Leitch IJ, Heslop-Harrison JS, 1992. Physical mapping of the 18S-5.8S-26S rRNA genes in barley byin situ hybridization. Genome 35: 1013–1018.
Lideikytė L, Pašakinskiené I, 2007. Genomic composition of amphiploid ×Festu Lolium braunii cultivars ‘Punia’ and ‘Rakopan’. Agriculture 94: 189–196.
Lideikytė L, Pašakinskienė I, Lemežienė N, Nekrošas S, Kanapeckas J, 2008. FISH assessment of ribosomal DNA sites in the chromosome sets ofLolium, Festuca andFestulolium. Agriculture 95: 116–124.
Lombard V, Delourme R, 2001. A consensus linkage map for rapeseed (Brassica napus L.): construction and integration of three individual maps from DH populations. Theor Appl Genet 103: 491–507.
Lysak MA, Berr A, Pecinka A, Schmidt R, McBreen K, Schubert I, 2006. Mechanisms of chromosome number reduction inArabidopsis thaliana and related Brassicaceae species. PNAS USA 103: 5224–5229.
Malik CP, Thomas PT, 1966. Karyotypic studies in someLolium andFestuca species. Caryologia 19: 167–196.
Maluszynska J, Heslop-Harrison JS, 1993. Physical mapping of rDNA loci inBrassica species. Genome 36: 774–781.
Mishima M, Ohmido N, Fukui K, Yahara T, 2002. Trends in site-number change of rDNA loci during polyploid evolution inSanguisorba (Rosaceae). Chromosoma 110: 550–558.
Mukai Y, Nakahara Y, Yamamoto M, 1993. Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescencein situ hybridization using total genomic and highly repeated DNA probes. Genome 36: 489–494.
Pedrosa-Harand A, de Almeida CCS, Mosiolek M, Blair MW, Schweizer D, Guerro M, 2006. Extensive ribosomal DNA amplification during Andean common bean (Phaseolus vulgaris L.) evolution. Theor Appl Genet 112: 924–933.
Pontes O, Neves N, Silva M, Lewis MS, Madlung A, Comai L, et al. 2004. Chromosomal locus rearrangements are a rapid response to formation of the allotetraploidArabidopsis suecica genome. PNAS USA 101: 18240–18245.
Raskina O, Belyayev A, Nevo E, 2004a. Activity of theEn/Spm-like transposons in meiosis as a base for chromosome repatterning in a small, isolated, peripheral population ofAegilops speltoides Tausch. Chrom Res 12: 153–161.
Raskina O, Belyayev A, Nevo E, 2004b. Quantum speciation inAegilops: Molecular cytogenetic evidence from rDNA cluster variability in natural populations. PNAS USA 101: 14818–14823.
Schubert I, Wobus U, 1985.in situ hybridization confirms jumping nucleolus organizing regions in Allium. Chromosoma 92: 143–148.
Shaked H, Kashkush K, Ozkan H, Feldman M, Levy AA, 2001. Sequence elimination and cytosine methylation are rapid and reproducible responses of the genome to wide hybridization and allopolyploids in wheat. Plant Cell 13: 1749–1759.
Thomas HM, 1981. The Giemsa C-band karyotypes of sixLolium species. Heredity 46: 263–267.
Thomas HM, Humphreys MO, 1991. Progress and potential of interspecific hybrids ofLolium andFestuca. J Agr Sci 117: 1–8.
Thomas HM, Harper JA, Meredith MR, Morgan WG, Thomas ID, Timms E, et al. 1996. Comparison of ribosomal DNA sites inLolium species by fluorescencein situ hybridization. Chrom Res 4: 486–490.
Thomas HM, Harper JA, Meredith MR, Morgan WG, King IP, 1997. Physical mapping of ribosomal DNA sites inFestuca arundinacea and related species byin situ hybridization. Genome 40: 406–410.
Thomas HM, Harper JA, Morgan WG, 2001. Gross chromosome rearrangements are occurring in an accession of the grassLolium rigidum. Chrom Res 9: 585–590.
Unfried I, Gruendler P, 1990. Nucleotide sequence of the 5.8S and 25S rRNA genes and the internal transcribed spacers fromArabidopsis thaliana. Nucl Acids Res 18: 4011.
Volkov RA, Borisjuk NV, Panchuk II, Schweizer D, Hemleben V, 1999. Elimination and rearrangement of parental rDNA in the allotetraploidNicotiana tabacum. Mol Biol Evol 16: 311–320.
Weiss H, Maluszynska J, 2000. Chromosomal rearrangement in autotetraploid plants ofArabidopsis thaliana. Hereditas 133: 255–261.
White SE, Habera LF, Wessler SR, 1994. Retrotransposons in the flanking regions of normal plant genes: a role forcopia-like elements in the evolution of the gene structure and expression. PNAS USA 91: 11792–11796.
Zwierzykowski Z, Kosmala A, Zwierzykowska E, Jones N, Jokoe W, Bocianowski J, 2006. Genome balance in six successive generations of the allotetraploidFestuca pratensis ×Lolium perenne. Theor Appl Genet 113: 539–547.
Zwierzykowski Z, Zwierzykowska E, Taciak M, Jones N, Kosmala A, Krajewski P, 2008. Chromosome pairing in allotetraploid hybrids ofFestuca pratensis ×Lolium perenne revealed by genomicin situ hybridization (GISH). Chrom Res 16: 575–585.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Książczyk, T., Taciak, M. & Zwierzykowski, Z. Variability of ribosomal DNA sites inFestuca pratensis, Lolium perenne, and their intergeneric hybrids, revealed by FISH and GISH. J Appl Genet 51, 449–460 (2010). https://doi.org/10.1007/BF03208874
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03208874