Abstract
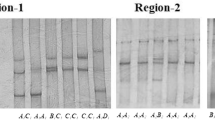
The contractile protein troponin I (TnI), a constituent protein of the troponin complex located on the thin filaments of striated muscle, is involved in inhibition of calcium-induced myosin AT Pase activity (and thus contraction). TnI-slow (slow-twitch skeletal muscle isoform, named TNNI1) and TnI-fast (fast-twitch skeletal muscle isoform, named TNNI2) are muscle-fiber-type-specific proteins, and expression of their genes may affect the composition of muscle fiber, thereby influencing the meat quality traits. Thus, the TnI genes are potential candidate genes for traits related to meat quality in animals. Association of 2 SNPs (EU743939:g.5174T>C in intron 4, and EU743939:g.8350C>A in intron 7) of theTNNI1 gene and a SNP (EU696779:g.1167C>T in intron 3) of theTNNI2 gene with 11 meat quality traits were studied on 334 Large White × Meishan F2 pigs. In theTNNI1 gene, g.5174T>C and g.8350C>A were found to be significantly associated with intramuscular fat content and meat color value of biceps femoris. The g.5174T>C also showed significant effects on meat color value and marbling score of longissimus dorsi, as well as pH of longissimus dorsi and semispinalis capitis. The g.1167C>T polymorphism in theTNNI2 gene affected significantly the pH of longissimus dorsi, meat color value of longissimus dorsi and semispinalis capitis, marbling score of longissimus dorsi, and intramuscular fat.
Similar content being viewed by others
References
Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statistical Society B 57: 289–300.
Bottinelli R, Reggiani C, 2000. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol 73: 195–262.
Calkins CR, Duston TR, Smith GC, Carpenter ZL, Davis GW, 1981. Relationship of fiber type composition to marbling and tenderness of bovine muscle. J Food Sci 46: 708–715.
Choe JH, Choi YM, Lee SH, Shin HG, Ryu YC, Hong KC, Kim BC, 2008. The relation between glycogen, lactate content and muscle fiber type composition, and their influence on postmortem glycolytic rate and pork quality. Meat Sci 80: 355–362.
Clark KA, McElhinny AS, Beckerle MC, Gregorio CC, 2002. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol 18: 637–706.
Corin SJ, Juhasz O, Zhu L, Conley P, Kedes L, Wade R, 1994. Structure and expression of the human slow twitch skeletal muscle troponin I gene. J Biol Chem 269: 10651–10659.
Mullen AJ, Barton PJ, 2000. Structural characterization of the human fast skeletal muscle troponin I gene (TNNI2). Gene 242: 313–320.
Ovilo C, Fernandez A, Rodriguez MC, Nieto M, Silio L, 2006. Association ofMC4R gene variants with growth, fatness, carcass composition and meat and fat quality traits in heavy pigs. Meat Sci 73: 42–47.
Pette D, Staron RS, 1997. Mammalian skeletal muscle fiber type transitions. Int Rev Cytol 170: 143–223.
Picard B, Lefaucheur L, Berri C, Duclos MJ, 2002. Muscle fibre ontogenesis in farm animal species. Reprod Nutr Dev 42: 415–431.
Polly P, Haddadi LM, Issa LL, Subramaniam N, Palmer SJ, Tay ESE, Hardeman EC, 2003. hMus TRD1α1 represses MEF2 activation of the troponin I slow enhancer. J Biol Chem 278: 36603–36610.
Ryu YC, Kim BC, 2005. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci 71: 351–357.
Ryu YC, Kim BC, 2006. Comparison of histochemical characteristics in various pork groups categorized by postmortem metabolic rate and pork quality. J Anim Sci 84: 894–901.
Schiaffino S, Reggiani C, 1996. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76: 371–423.
Varona L, Gomez-Raya L, Rauw WM, Noguera JL, 2005. A simulation study on the detection of causal mutations from F2 experiments. J Anim Breed Genet 122: 30–36.
Xiong YZ, Deng CY, 1999. Principle and method of swine testing. Chinese Agricultural Press, Beijing.
Zuo B, Yang H, Lei MG, Li FE, Deng CY, Jiang SW, Xiong YZ, 2007. Association of the polymorphism inGYS1 andACOX1 gene with meat quality traits in pigs. Animal 1: 1243–1248.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, H., Xu, Z.Y., Lei, M.G. et al. Association of 3 polymorphisms in porcine troponin I genes (TNNI1 andTNNI2) with meat quality traits. J Appl Genet 51, 51–57 (2010). https://doi.org/10.1007/BF03195710
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03195710