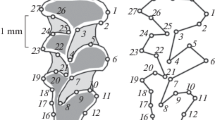
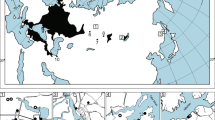
Abstract
Research into the geographical pattern of tooth size in the red fox,Vulpes vulpes (Linnaeus, 1758) in the Holarctic was conducted on a sample of 3806 skulls belonging to 41 fox populations. The Nearctic was represented by 948 specimens (249 females, 359 males, 340 specimens of unknown sex) belonging to 13 populations, whereas the Palearctic was represented by 2858 red foxes (1034 females, 1256 males, 568 specimens of unknown sex) from 32 populations. In the Nearctic, the largest foxes live on Kodiak Island (V. v. harrimani) and the Kenai Peninsula (V. v. kenaiensis), while the smallest ones live in California (V. v. necator) and Georgia (V. v. fulvus). In the Palearctic, the largest foxes come from the Far East (V. v. jakutensis, V. v. beringiana, V. v. tobolica), while the smallest are from the southern borders of the Eurasian range (V. v. pusilla, V. v. barbara, V. v. arabica). In both the Palearctic and Nearctic, tooth size in the fox varies depending on the geo-climatic factors. The fox’s tooth size confirms the general basis of Bergmann’s rule. In the Palearctic, specimens with larger teeth occur in cooler habitats with greater seasonality. These are first and foremost Northern and Far Eastern populations. In the Nearctic, tooth size in red foxes depends on the temperature and humidity of their habitat. Competition within the species and between species has important impact on the variation and dimorphism of tooth size in the red fox. Both in the Nearctic and Palearctic, red foxes from regions of sympatric co-occurrence with other closely relatedVulpes species, are more sexually dimorphic in terms of tooth size than red foxes from allopatric regions. Analysis of morphological distance on the basis of the size of dental characteristics shows, that in the Palearctic, the foxes from India (V. v. pusilla), while in the Nearctic, the population from Kodiak Island (V. v. harrimani) are most distant from the remaining populations. Geographic barriers such as the Bering Strait, Parry Channel, Mackenzie River, Kolyma and Omolon River systems have had a critical impact on red fox evolution. The most likely place for the evolution and diversification of the phyletic lineVulpes vulpes seems to be the Middle East region.
Similar content being viewed by others
References
Abbott R. J., Smith L. C., Milne R. I., Crawford R. M. M., Wolf K. and Balfour J. 2000. Molecular analysis of plant migration and refugia in the Arctic. Science 289: 1343–1346.
Anderson E. 1970. Quternary evolution of the genusMartes (Carnivora, Mustelidae). Acta Zoologica Fennica 130: 1–132.
Ansorge H. 1994. Intrapopular skull variability in the red fox,Vulpes vulpes (Mammalia: Carnivora: Canidae). Zoologische Abhandlungen Statliches Museum für Tierkunden Dresden 48: 103–123.
Aristov A. A. and Baryshnikov G. F. 2001. Mlekopitayushchie fauny Rossii i sopredel’nykh territorii. Khishchnye i lastonogie. Zoologicheskii Institut RAN, Sankt-Peterburg 169: 1–560. [In Russian]
Ashton K. L., Tracy M. C. and de Queiroz A. 2000. Is Bergmann rule valid for mammals? The American Naturalist 156: 390–415.
Aubry K. B. 1984. The recent history and present distribution of the red fox in Washington. Northwest Science 58: 69–79.
Best T. L. 1981. Relationship between ecographic and morphologic variation in the agilo kangaroo rat (Dipodomys agilis) in Baja California, Mexico. Bulletin of the Southern California Academy of Sciences 80: 60–69.
Bonis L. de, Peigné S., Likius A., Mackaye H. T., Vignaud P. and Brunet M. 2007. The oldest African fox (Vulpes riffautae n. sp., Canidae, Carnivora) recovered in late Miocene deposits of the Djurab desert, Chad. Naturwissenschaften 94: 575–580.
Brown W. L. and Wilson E. O. 1956. Character displacement. Systematic Zoology 7: 49–64.
Brunhoff C., Galbreath K. E., Fedorov V., Cook J. A. and Jaarola M. 2003. Holarctic phylogeography of the root vole (Microtus oeconomus): implications for late Quaternary biogeography of high latitudes. Molecular Ecology 12: 957–968.
Butler P. M. 1985. Homologies of molar cusps and crests, and their bearing on assessments of rodent phylogeny. [In: Evolutionary relationships among rodents P.Luckett, ed]. Plenum Publishing Corporation, New York: 381–401.
Carraway L. N. and Verts B. J. 1994. Relationship of mandibular morphology to relative bite force in someSorex from western North America. Special Publication Carnegie Museum of Natural History 18: 201–210.
Cavallini P. 1995. Variation in body size of the red fox. Annales Zoologici Fennici 32: 421–427.
Churcher C. S. 1960. Cranial variation in the North American red fox. Journal of Mammalogy 41: 349–360.
Degerböl M. 1933. Danmarks pattedyr i fortiden i sammenligning med recente former. Videnskabelige Meddelelser fra Dansk naturhistorisk Forening i Kiøbenhavn 96: 353–641. [In Danish]
Davis S. 1977. Size variation of the fox,Vulpes vulpes in the palearctic region today, and in Israel during late Quaternary. Journal of Zoology, London 182: 343–351.
Dayan T. and Simberloff D. 1994. Character displacement, sexual dimorphism, and morphological variation among British and Irish mustelids. Ecology 75: 1063–1073.
Dayan T. and Simberloff D. 1998. Size pattern among competitors: ecological displacement and character release in mammals, with special reference to island populations. Mammal Review 28: 99–124.
Dayan T., Simberloff D., Tchernov E. and Yom-Tov Y. 1989a. Inter- and intraspecific character displacement in mustelids. Ecology 70: 1526–1539.
Dayan T., Simberloff D., Tchernov E. and Yom-Tov Y. 1990. Feline canines: community-wide character displacement among the small cats of Israel. The American Naturalist 136: 39–60.
Dayan T., Simberloff D., Tchernov E. and Yom-Tov Y. 1992a. Canine carnassials: character displacement in the wolves, jackals and foxes of Israel. Biological Journal of the Linnean Society 45: 315–331.
Dayan T., Tchernov E., Simberloff D. and Yom-Tov Y. 1992b. Tooth size: function and coevolution in carnivore guilds. [In: Structure, function and evolution of teeth. P. Smith and E. Tchernov, eds]. Freund Publishing House Ltd., London and Tel Aviv: 215–222.
Dayan T., Tchernov E., Yom-Tov Y. and Simberloff D. 1989b. Ecological character displacement in Saharo-Arabian Vulpes: outfoxing Bergmann’s rule. Oikos 55: 263–272.
Dayan T., Wool D. and Simberloff D. 2002. Variation and covariation of skulls and teeth: modern carnivores and interpretation of fossil mammals. Paleobiology 28: 508–526.
Douma-Petridou E. and Ondrias J. C. 1980. Contribution to the knowledge ofVulpes vulpes L. (Mammalia, Carnivora) from Achaia, Peloponnesus, Greece, with a description of a new subspecies. Biologia Gallo Hellenica 9: 207–217.
Ebenman B. 1986. Sexual size dimorphism in the great titParus major in relation to the number coexisting congeners. Oikos 47: 355–359.
Ebersbach H. and Stubbe M. 1994. Development of body weight and reproduction in some marten-like mammals. Beiträge zur Jagd- und Wildforschung 19: 197–212.
Ehrich D., Fedorov V. B., Stenseth N. C., Krebs J. C. and Kenney A. 2000. Phylogeography of mitochondrial DNA (mtDNA) diversity in North American collared lemmings (Dicrostonyx groenlandicus). Molecular Ecology 9: 329–337.
Fedorov V. B., Fredga K. and Jarrell G. H. 1999a. Mitochondrial DNA variation and the evolutionary history of chromosome races of collared lemmings (Dicrostonyx) in the Eurasian Arctic. Journal of Evolutionary Biology 12: 134–145.
Fedorov V. B., Goropashnaya A., Jaarola M. and Cook J. A. 2003. Phylogeography of lemmings (Lemmus): no evidence for postglacial colonization of Arcitc from Beringian refugium. Molecular Ecology 12: 725–732.
Fedorov V. B., Goropashnaya A., Jarrell G. H. and Fredga K. 1999b. Phylogeographic structure and mitochondrial DNA variation in true lemmings (Lemmus) from the Eurasian Arctic. Biological Journal of the Linnean Society 66: 357–371.
Fedorov V. B. and Stenseth N. C. 2002. Multiple glacial refugia in the North American Arctic: inference from phylogeography of the collared lemming (Dicrostonyx groenlandicus). Proceedings of the Royal Society of London B 269: 2071–2077.
Fisher D. O. and Dickman C. R. 1993. Diets of insectivorous marsupials in arid Australia: selection for prey type, size or hardness? Journal of Arid Environments 25: 397–410.
Frafjord K. and Stevy I. 1998. The red fox in Norway: morphological adaptation or random variation in size? Zeitschrift für Säugetierkunde 63: 16–25.
Frenzel B. 1968. The Pleistocene vegetation of northern Eurasia. Science 161: 637–649.
Futuyama D. J. 1998. Evolutionary biology. 3rd edition. Sinauer, Sunderland, Mass: 1–828.
Galbreath K. E. and Cook J. 2004. Genetic consequences of Pleistocene glaciations for the tundra vole (Microtus oeconomus) in Beringia. Molecular Ecology 13: 135–148.
Geffen E., Mercure A., Girman D. J., Macdonald D. W. and Wayne R. K. 1992. Phylogenetic relationships of fox-like canids: mitochondrial DNA restriction fragment, site and cytochromeb sequence analyses. Journal of Zoology, London 228: 27–39.
Geist V. 1987. Bergmann’s rule is invalid. Canadian Journal of Zoology 65: 1035–1038.
Gingerich P. D. 1976. Cranial anatomy and evolution of early Tertiary Plesiadapidae (Mammalia, Primates). Papers on Paleontology, Museum of Paleontology, University of Michigan 15: 1–145.
Gingerich P. D. 1977. Patterns of evolution in the mammalian fossil record. [In: Patterns of evolution as illustrated by the fossil record. A. Hallam, ed]. Elsevier Scientific, Amsterdam: 469–500.
Gingerich P. D. and Simons E. L. 1977. Systematics, phylogeny, and evolution of early Eocene Adapidae (Mammalia, Primates) in North America. Contributions from the Museum of Paleontology. The University of Michigan 24: 245–279.
Gingerich P. D. and Winkler D. A. 1979. Patterns of variation and correlation in the dentition of the red fox,Vulpes vulpes. Journal of Mammalogy 60: 691–704.
Gittleman J. L. and Van Valkenburgh B. 1997. Sexual dimorphism in the canines and skulls of carnivores: effects of size, phylogeny, and behavioural ecology. Journal of Zoology, London 242: 97–117.
Gortázar C., Travaini A. and Delibes M. 2000. Habitat-related microgeographic body size variation in two Mediterranean populations of red fox (Vulpes vulpes). Journal of Zoology, London 250: 335–338.
Goszczyński J. 1995. [The fox]. Monografia przyrodniczo-łowiecka. Oikos, Warszawa: 1–137. [In Polish]
Gould S. J. 1975. On the scaling of tooth size in mammals. American Zoologist 15: 351–362.
Hall E. and Kelson K. 1959. The Mammals of North America. The Ronald Press Company, New York: 1–1083.
Haltenorth T. and Roth H. H. 1968. Short review of the biology and ecology of the red foxCanis (Vulpes)vulpes Linnaeus 1758. Säugetierkundliche Mitteilungen 16: 339–352.
Harrison D. L. and Bates P. J. J. 1991. The mammals of Arabia. Harrison Zoological Museum Publication, Severoaks, Kent: 1–354.
Hell P., Paule L., Sevčenko L. S., Danko S., Panigaj L. and Vítaz V. 1989. Craniometrical investigation of the red fox (Vulpes vulpes) from the Slovak Carpathians and adjacent lowlands. Folia Zoologica 38: 139–155.
Hewitt G. M. 1996. Some genetic consequences of ice ages, and their role in divergence and speciation. Biological Journal of the Linnean Society 58: 247–276.
Holmes T. and Powell R. A. 1994. Morphology, ecology and the evolution of sexual dimorphism in North American Martes. [In: Martens, sables, and fishers: biology and conservation. S. W. Buskirk, A. S. Harestad, M. G. Raphael and R. A. Powell, eds]. Comstock Publishing Associates, Ithaca, New York, USA: 72–84.
Hopkins D. M. (ed) 1967. The Bering Land Bridge. Stanford University Press, California: 1–495.
Huson L. W. and Page R. J. C. 1979. A comparison of fox skulls from Wales and South-East England. Journal of Zoology, London 187: 465–470.
Huson L. W. and Page R. J. C. 1980. Multivariate geographical variation of the red fox (Vulpes vulpes) in Wales. Journal of Zoology, London 191: 453–459.
James F. C. 1970. Geographic size variation in birds and its relationship to climate. Ecology 51: 365–390.
Jiménez J. E., Yáńez J. L., Tabilo E. L. and Jaksić F. M. 1995. Body size of Chilean foxes: a new pattern in light of new data. Acta Theriologica 40: 321–326.
Johnson D. D. P. and Macdonald D. W. 2001. Why are group-living badgers (Meles meles) sexually dimorphic? Journal of Zoology, London 255: 199–204.
Kaleta T. 1998. Wild dogs and hyaenas. Wiedza Powszechna, Warsaw: 1–219. [In Polish]
Kamler J. F. and Ballard W. B. 2002. A review of native and nonnative red foxes in North America. Wildlife Society Bulletin 30: 370–379.
Kay R. F. 1975. The functional adaptations of primate molar teeth. American Journal of Physical Anthropology 43: 195–216.
Kennedy M. L. and Lindsay S. L. 1984. Morphological variation in the raccoon,Procyon lotor, and its relationship to genic and environmental variation. Journal of Mammalogy 65: 195–205.
Kingdon J. 1991. Arabian Mammals. A Natural History. Academic Press, Harcourt Brace Jovanovich, London: 1–279.
Klein R. G. 1986. Carnivore size and Quternary climatic change in southern Africa. Quaternary Research 26: 153–170.
Klein R. G. and Scott K. 1989. Glacial/interglacial size variation in fossil spotted hyaenas (Crocuta crocuta) from Britain. Quaternary Research 32: 88–95.
Kolb H. H. 1978. Variation in the size of foxes in Scotland. Biological Journal of the Linnean Society 10: 291–304.
Kolb H. H. and Hewson R. 1974. The body size in the red fox (Vulpes vulpes) in Scotland. Journal of Zoology, London 173: 253–255.
Kowalczyk R., Jędrzejewska B. and Zalewski A. 2003. Annual and circadian activity patterns of badgers (Meles meles) in Białowieża Primeval Forest (eastern Poland) compared with other Palearctic populations. Journal of Biogeography 30: 463–472.
Krasińska M., Szuma E., Kobryńczuk F. and Szara T. 2008. Morphometric variation of the skull during postnatal development in the Lowland European bison,Bison bonasus bonasus. Acta Theriologica 53: 193–216.
Kurtén B. 1967. Some quantitative approaches to dental microevolution. Journal of Dental Research 40: 817–828.
Kurtén B. and Anderson E. 1980. Pleistocene mammals of North America. Columbia University Press, New York: 1–442.
Larivière S. and Pasitschniak-Arts M. 1996.Vulpes vulpes. Mammalian Species 537: 1–11.
Legendre S. and Roth C. 1988. Correlation of carnassial tooth size and body weight in recent carnivores (Mammalia). Historical Biology 1: 85–98.
Lidicker W. Z. Jr 1991. Introduced mammals in California. [In: Biogeography of Mediterranean Invasions. R. H. Groves and F. di Castri, eds]. Cambridge University Press, Cambridge: 263–271.
Lindsted S. L. and Boyce M. C. 1985. Seasonality, body size and survival time in mammals. The American Naturalist 125: 873–878.
Linhart S. B. 1968. Dentition and pelage in the juvenile red fox (Vulpes vulpes). Journal of Mammalogy 49: 526–528.
Lloyd H. G. 1981. The red fox. B. T. Batsford Ltd, London: 1–285.
Lüps P. and Roper T. J. 1988. Tooth size in the European badger (Meles meles) with special refernce to sexual dimorphism, diet and intraspecific aggression. Acta Theriologica 33: 21–33.
MacArthur R. H. and Wilson E. O. 1967. The theory of island biogeography. Princeton University Press, Princeton, New Jersey: 1–203.
Macdonald D. W., Courtenay O., Forbes S. and Mathews F. 1999. The red fox (Vulpes vulpes) in Saudi Arabia: loose-knit groupings in the absence of territoriality. Journal of Zoology, London 249: 383–391.
MacNab B. K. 1971. On the ecological significance of Bergmann’s rule. Ecology 52: 845–854.
Meiri S. and Dayan T. 2003. On the validity of Bergmann’s rule. Journal of Biogeography 30: 331–351.
Meiri S., Dayan T. and Simberloff D. 2004. Carnivores, biases and Bergmann’s rule. Biological Journal of the Linnean Society 81: 579–588.
Meiri S., Dayan T. and Simberloff D. 2005. Variability and sexual size dimorphism in Carnivores: testing the niche variation hypothesis. Ecology 86: 1432–1440.
Mendelssohn H., Yom-Tov Y., Ilany G. and Meninger D. 1987. On the occurrence of Blanford’s foxVulpes cana Blanford 1877, in Israel and Sinai. Mammalia 51: 459–462.
Merriam C. H. 1900. Preliminary revision of the North American red foxes. Process Washington Academy of Sciences 2: 661–676.
Mikesic D. G. and LaRue C. T. 2003. Recent status and distribution of red foxes (Vulpes vulpes) in northeastern Arizona and southeastern Utah. The Southwestern Naturalist 48: 624–634.
Mugaas J. N. and Seidensticker J. 1993. Geographic variation of lean body mass and a model of its effect on the capacity of the raccoon to fatten and fast. Bulletin of the Florida Museum of Natural History Biological Sciences 36: 85–107.
Murphy E. C. 1985. Bergmann’s rule, seasonality, and geographic variation in body size of house sparrows. Evolution 39: 1327–1334.
Patterson B. D. 1983. Grasshopper mandibles and the niche variation hypothesis. Evolution 37: 375–388.
Pengilly D. 1984. Developmental versus functional explanations for patterns of variability and correlation in the dentitions of foxes. Journal of Mammalogy 65: 34–43.
Plavcan J. M. 2004. Sexual selection, measures of sexual selection and sexual dimorphism in primates. [In: Sexual selection in primates: new and comparative perspectives. P. M. Kappeler and C. D. Van Schaik, eds]. Cambridge University Press, Cambridge: 230–252.
Polly P. D. 1998. Variability in mammalian dentitions: size-related bias in the coefficient of variation. Biological Journal of the Linnean Society 64: 83–99.
Polly P. D. 2003a. Paleophylogeography ofSorex araneus (Insectivora, Soricidae): molar shape as a morphological marker for fossil shrews. Mammalia 68: 233–243.
Polly P. D. 2003b. Paleophylogeography: the tempo of geographic deifferentiation in marmots (Marmota). Journal of Mammalogy 84: 369–384.
Polly P. D. 2005. Development and phenotypic correlations: the evolution of tooth shape inSorex araneus. Evolution & Development 7: 24–41.
Pucek Z. 1965. Seasonal and age changes in the weight of internal organs of shrews. Acta Theriologica 10: 369–438.
Pulliainen E., Rantanen A. V. and Salo L. J. 1972. On the carnassial tooth cusps in recent red foxes (Vulpes vulpes L.) in Finland and Denmark. Scandinavian Journal of Dental Research 80: 322–326.
Raia P. and Meiri S. 2006. The Island rule in large mammals: paleontology meets ecology. Evolution 60: 1731–1742.
Ralls K. and Harvey P. H. 1985. Geographic variation in size and sexual dimorphism of North American weasels. Biological Journal of the Linnean Society 25: 119–167.
Rausch R. L. 1963. Geographic variation in size in North American brown bears, Ursus arctos L., as indicated by condylobasal length. Canadian Journal of Zoology 41: 33–45.
Ritke M. E. and Kennedy M. L. 1988. Intraspecific morphologic variation in the raccoon (Procyon lotor) and its relationship to selected environmental variables. The Southwestern Naturalist 33: 295–314.
Rosenzweig M. L. 1968. The strategy of body size in mammalian carnivores. The American Midland Naturalist 80: 299–315.
Sage R. D. and Wolf J. O. 1986. Pleistocene glaciations, fluctuating ranges, and low genetic variability in a large mammal (Ovis dalli). Evolution 40: 1092–1093.
Samuel D. E. and Nelson B. B. 1982. Foxes. [In: Wild mammals of North America: biology, management, and economics. J. L. Chapman and G. A. Feldhamer, eds]. The John Hopkins University Press, Baltimore, Maryland: 475–490.
Savage R. J. G. and Russell D. E. 1983. Mammalian paleofaunas of the world. Addison — Wesley Publishing Co., London: xvii-432.
Shevchenko L. S. 1987. [Craniometric indices in red fox of European part USSR]. Vestnik Zoologii 1987: 63–71. [In Russian]
Simms D. A. 1979. North American weasels: resource utilization and distribution. Canadian Journal of Zoology 57: 504–520.
Simonsen V., Pertoldi C., Madsen A. B. and Loeschecke V. 2003. Genetic differentiation of foxes (Vulpes vulpes) analysed by means of craniometry and isozymes. Journal for Nature Conservation 11: 109–116.
Snell R. R. and Cunnison K. M. 1983. Relationship of geographic variation in the skull of Microtus pennsylvanicus to climate. Canadian Journal of Zoology 61: 1232–1241.
Statistica.PL 1997. STATISTICA PL dla Windows. StatSoft version 6.0 Polska Sp. z o.o.
Storm G. L., Andrews R. D., Phillips R. L., Bishop R. A., Siniff D. B. and Tester J. R. 1976. Morphology, reproduction, dispersal, and mortality of Midwestern red fox populations. Wildlife Monographs 49: 1–82.
Suchentrunk F. 1994. Non-metrical polymorphism of the first lower premolar (P3) in Austrian brown hares (Lepus europaeus): a study on regional differentiation. Journal of Zoology, London 232: 79–91.
Suchentrunk F. 2000. Epigenetic dental asymmetry of Israeli hares: developmental stability along an environmental gradient. Israel Journal of Zoology 46: 103–118.
Suchentrunk F. 2004. Phylogenetic relationships between Indian and Burmese hares (Lepus nigricollis and L. peugensis) inferred from epigenetic dental characters. Mammalian Biology 69: 28–45.
Suchentrunk F., Alkon P. U., Wiling R. and Yom-Tov Y. 2000. Epigenetic dental variability of Israeli hares (Lepus sp.): ecogenetic or phylogenetic causation? Journal of Zoology, London 252: 503–515.
Suchentrunk F. and Flux J. E. C. 1996. Minor dental traits in East African cape hares and savanna hares (Lepus capensis andLepus victoriae): A study of intra- and interspecific variability. Journal of Zoology, London 238: 495–511.
Szuma E. 1999. Dental abnormalities in the red foxVulpes vulpes from Poland. Acta Theriologica 44: 393–412.
Szuma E. 2000. Variation and correlation patterns in the dentition of the red fox from Poland. Annales Zoologici Fennici 37: 113–127.
Szuma E. 2003. Microevolutionary trends in the dentition of the Red fox (Vulpes vulpes). Journal of Zoological Systematics and Evolutionary Research 41: 47–56.
Szuma E. 2004. Evolutionary implications of morphological variations in the lower carnassial of Red FoxVulpes vulpes. Acta Theriologica 49: 433–447.
Szuma E. 2007. Geography of dental polymorphism in the red foxVulpes vulpes and its evolutionary implications. Biological Journal of the Linnean Society 90: 61–84.
Szuma E. 2008a. Geography of sexual dimorphism in the tooth size of the red foxVulpes vulpes (Mammalia, Carnivora). Journal of Zoological Systematic and Evolutionary Research 46: 73–81.
Szuma E. 2008b. Geographic variation of tooth and skull size in the arctic foxVulpes (Alopex)lagopus. Annales Zoologici Fennici 45: 185–199.
Tedford R. H., Taylor B. E. and Wang X. 1995. Phylogeny of the Caninae (Carnivora: Canidae): the living taxa. American Museum Novitates 3146: 1–37.
Thoréén S., Lindenfors P. and Kappeler P. M. 2006. Phylogenetic analyses of dimorphism in primates: evidence for stronger selection on canine size than on body size. American Journal of Physical Anthropology 130: 50–59.
Travaini A. and Delibes M. 1995. Weight and external measurements of red foxes (Vulpes vulpes) from SW Spain. Zeitschrift für Säugetierkunde 60: 121–123.
Van Gelder R. G. 1968. The genusConepatus (Mammalia, Mustelidae): variation within a population. American Museum Novitates 2322: 1–37.
Van Valen L. M. 1965. Morphological variation and the width of the ecological niche. The American Naturalist 99: 377–390.
Van Valkenburgh B. 1989. Carnivore dental adaptations and diet: a study of trophic diversity within guilds. [In: Carnivore behavior, ecology, and evolution. J. L. Gittleman, ed]. Chapmann and Hall, London, Cornell University Press, New York: 410–435.
Van Valkenburgh B. 1990. Skeletal and dental predictors of body mass in carnivores. [In: Body size in mammalian paleobiology: Estimation and biological implications. J. Damuth and B. J. MacFadden, eds]. Cambridge University Press, Cambridge: 181–205.
Voigt D. R. 1987. Red fox. [In: Wild furbearer management and conservation in North America. M. Nowak, J. A. Baker, M. E. Obbard and B. Malloch, eds]. Ontario Ministry of Natural Resources, Ontario: 379–392.
Waltari E., Demboski J. R., Klein D. R. and Cook J. A. 2004. A molecular perspective on the historical biogeography of the northern high latitudes. Journal of Mammalogy 85: 591–600.
Wang X., Tedford R. H., Van Valkenburgh B. and Wayne R. K. 2004. Ancestry history, molecular systematic, and evolutionary ecology of Canidae. [In: Biology and conservation of wild canids. D. W. Macdonald and C. Sillero-Zubiri, eds]. Oxford University Press, Oxford, New York: 39–54.
Wenink P. W., Baker A. J., Rosner H.-U. and Tilanus M. G. J. 1996. Global mitochondrial DNA phylogeography of holarctic breeding dunlins (Calidris alpine). Evolution 50: 318–330.
Wilson D. E. and Ruff S. (eds). 1999. Smithsonian Book of North American Mammals. Smithsonian Institution Press, Washington: 1–750.
Whittaker R. 1998. Island biogeography — ecology, evolution and conservation. Oxford University Press, Oxford: 1–285.
Wolsan M. 1985. Variation and asymmetry in the dentition of the pine and stone martens (Martes martes and M. foina) from Poland. Acta Theriologica 30: 79–114.
Wolsan M. 1989. Dental polymorphism in the genus Martes (Carnivora: Mustelidae) and its evolutionary significance. Acta Theriologica 34: 545–593.
WorldClimate. 1996–2004. Buttle and Tuttle Ltd.: www.worldclimate.com
Yablokov A. V. 1974. Variability of mammals. Amerind Publishing Co., New Delhi: 1–350.
Yom-Tov Y. and Geffen E. 2006. Geographic variation in body size: the effect ambient temperature and precipitation. Oecologia 148: 213–218.
Yudin V. G. 1986. Lisitsa Dal’nego Vostoka SSSR. Vladivostok: Akademiya Nauk SSSR. Dal’nevostochnyi Nauchnyi Tsentr Biologo-Pochvennoyi Institut, Vladivostok: 1–284. [In Russian]
Zeveloff S. I. and Boyce M. S. 1988. Body size patterns in North American mammal faunas. [In: Evolution of life histories of mammals, theory and pattern. M. S. Boyce, ed]. Yale University Press, New Haven, CT: 123–146.
Author information
Authors and Affiliations
Additional information
Associate editor was Krzysztof Schmidt.
An Erratum for this chapter can be found at http://dx.doi.org/10.1007/BF03193142
Rights and permissions
About this article
Cite this article
Szuma, E. Evolutionary and climatic factors affecting tooth size in the red foxVulpes vulpes in the Holarctic. Acta Theriol 53, 289–332 (2008). https://doi.org/10.1007/BF03195193
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03195193