Abstract
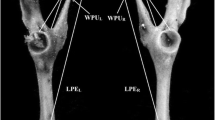
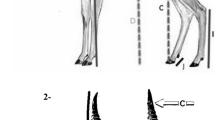
Many sexually-selected structures are variable and positively allometric relative to body size. For the western martenMartes caurina Merriam, 1890 from Vancouver Island, British Columbia, we investigated variation in the baculum compared with other bones and allometry of bacular to body size. Carcass length did not differ, and humeral and mandibular lengths differed little (< 1 and 2%, respectively), between age groupings < 1 and ≥ 1 yr old. In contrast, bacular length increased by 16%, and thickness by 29% (mid shaft) and 86% (basal) between those groupings, and thickness and mass continued to grow after the second year of life. Controlling for body size, bacular size varied more than humeral or mandibular size (CV for linear variables ∼4–8% for baculum, ∼2% for humerus or mandible). Some positive static allometry of bacular size to body size was found, but correlations between bacular and body size were weak (r=0.3–0.4). So penile size as related to bacular size could be a reliable but imprecise quality indicator during copulation. Weak polygamy (serial promiscuity), complex copulatory mechanisms, and high energetic costs of reproduction, likely select for multiple cues in mate-choice by females, not just penile cues affected by bacular size or shape.
Similar content being viewed by others
References
Anderson M. J., Dixson A. S. and Dixson A. F. 2006. Mammalian sperm and oviducts are sexually selected: evidence for co-evolution. Journal of Zoology, London 270: 682–686.
Andersson M. 1994. Sexual selection. Princeton University Press, Princeton, New Jersey: 1–599.
Arata A. A., Negus N. C. and Downs M. S. 1965. Histology, development, and individual variation of complex muroid bacula. Tulane Studies in Zoology 12: 51–64.
Arnqvist G. 1997. The evolution of animal genitalia: distinguishing between hypotheses by single species studies. Biological Journal of the Linnean Society 60: 365–379.
Arnqvist G. and Danielsson I. 1999. Copulatory behavior, genital morphology, and male fertilization success in water striders. Evolution 53: 147–156.
Artimo A. 1969. The baculum in the wood lemming,Myopus schisticolor (Lilljeb.), in relation to sexual status, size and age. Annales Zoologici Fennici 6: 335–344.
Ashbrook F. G. and Hanson K. B. 1930. The normal breeding season and gestation period of martens. United States Department of Agriculture Circular 107: 1–6.
Baryshnikov G. F. and Abramov A. V. 1997. Structure of baculum (os penis) in Mustelidae (Mammalia, Carnivora). Communication 1. Zoologicheskii Zhurnal 76: 1399–1410. [In Russian with English summary]
Baryshnikov G. F. and Abramov A. V. 1998. Structure of baculum (os penis) in Mustelidae (Mammalia, Carnivora). Communication 2. Zoologicheskii Zhurnal 77: 231–236. [In Russian with English summary]
Baryshnikov G. F., Bininda-Emonds O. R. P. and Abramov A. V. 2003. Morphological variability and evolution of the baculum (os penis) in Mustelidae (Carnivora). Journal of Mammalogy 84: 673–690.
Bertin A. and Fairbairn D. J. 2007. The form of sexual selection on male genitalia cannot be inferred from within-population variance and allometry — a case study inAquarius remigis. Evolution 61: 825–837.
Bonduriansky R. 2007. Sexual selection and allometry: a critical reappraisal of the evidence and ideas. Evolution 61: 838–849.
Bonduriansky R. and Day T. 2003. The evolution of static allometry in sexually selected traits. Evolution 57: 2450–2458.
Brennan P. L. R., Prum R. O., McCracken K. G., Sorenson M. D., Wilson R. E. and Birkhead T. R. 2007. Coevolution of male and female genital morphology in waterfowl. PLoS ONE 2(5): e418.
Burt W. H. 1960. Bacula of North American mammals. Miscellaneous Publications, Museum of Zoology, University of Michigan 113: 1–75.
Buskirk S. W. and Ruggiero L. F. 1994. American marten. [In: The scientific basis for conserving forest carnivores: considerations for management. L. F. Ruggiero, K. B. Aubry, S. W. Buskirk, L. J. Lyon and W. J. Zielinski, eds]. United States Forest Service General Technical Report RM 254: 7–37.
Carr S. M. and Hicks S. A. 1997. Are there two species of marten in North America? Genetic and evolutionary relationships withinMartes. [In:Martes: taxonomy, ecology, techniques, and management. G. Proulx, H. N. Bryant and P. M. Woodard, eds]. Provincial Museum of Alberta, Edmonton, Alberta: 15–28.
Clark T. W., Anderson E., Douglas C. and Strickland M. 1987.Martes americana. Mammalian Species 289: 1–8.
Cock A. G. 1966. Genetical aspects of metrical growth and form in animals. Quarterly Review of Biology 41: 131–190.
Cuervo J. J. and Møller A. P. 2001. Components of phenotypic variation in avian ornamental and non-ornamental feathers. Evolutionary Ecology 15: 53–72.
Dagg A. I., Leach D. and Sumner-Smith G. 1975. Fusion of the distal femoral epiphysis in male and female marten and fisher. Canadian Journal of Zoology 53: 1514–1518.
Dix L. M. and Strickland M. A. 1986. Use of tooth radiographs to classify martens by sex and age. Wildlife Society Bulletin 14: 275–279.
Dixson A. F. 1995. Baculum length and copulatory behaviour in carnivores and pinnipeds (Grand Order Ferae). Journal of Zoology, London 235: 67–76.
Douglas C. W. and Strickland M. A. 1987. Fisher. [In: Wild furbearer management and conservation in North America. M. Novak, J. A. Baker, M. E. Obbard and B. Malloch, eds]. Ontario Trappers’ Association, Toronto, Ontario: 510–529.
Dyck M. G., Bourgeois J. M. and Miller E. H. 2004. Growth and variation in the bacula of polar bears (Ursus maritimus) in the Canadian Arctic. Journal of Zoology, London 264: 105–110.
Eberhard W. G. 1985. Sexual selection and animal genitalia. Harvard University Press, Cambridge, Massachusetts: 1–244.
Eberhard W. G. 1996. Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton, New Jersey: 1–520.
Eberhard W. G., Huber B. A., Rodriguez-S. R. L., Briceño R. D., Salas I. and Rodriquez V. 1998. One size fits all? Relationships between the size and degree of variation in genitalia and other body parts in twenty species of insects and spiders. Evolution 52: 415–431.
Eberhard W., Rodriguez R. L. and Polihronakis M. (in press). The functional significance of allometry: comments on Bertin & Fairbairn 2007. Evolution.
Elder W. H. 1951. The baculum as an age criterion in mink. Journal of Mammalogy 32: 43–50.
Emlen S. T. and Oring L. W. 1977. Ecology, sexual selection, and the evolution of animal mating systems. Science 197: 215–223.
Ewer R. F. 1968. Ethology of mammals. Plenum, New York: 1–418.
Fay F. H. 1982. Ecology and biology of the Pacific walrus,Odobenus rosmarus divergens Illiger. U. S. Department of the Interior, Fish and Wildlife Service, Washington, D. C.: 1–279.
Ferguson S. H. and Larivière S. 2004a. Are long penis bones an adaptation to high latitude snowy environments? Oikos 105: 255–267.
Ferguson S. H. and Larivière S. 2004b. Is mustelid life history different? [In: Martens and fishers (Martes) in human-altered environments: an international perspective. D. J. Harrison, A. K. Fuller and G. Proulx, eds]. Kluwer Academic Publishers, Norwell, Massachusetts: 2–19.
Friley C. E. J. Jr 1949a. Use of the baculum in age determination of Michigan beaver. Journal of Mammalogy 30: 261–267.
Friley C. E. J. Jr 1949b. Age determination by use of the baculum in the river otter,Lutra c. canadensis Schreber. Journal of Mammalogy 30: 102–110.
Giannico G. R. and Nagorsen D. W. 1989. Geographic and sexual variation in the skull of Pacific Coast marten (Martes americana). Canadian Journal of Zoology 67: 1386–1393.
Gould S. J. 1974. The evolutionary significance of “bizarre” structures: antler size and skull size in the “Irish elk”,Megaloceros giganteus. Evolution 28: 191–220.
Grant J. and Hawley A. 1991. Some observations on the mating behaviour of captive American pine martensMartes americana. Acta Theriologica 41: 439–442.
Green A. J. 1992. Positive allometry is likely with mate choice, competitive display and other functions. Animal Behaviour 43: 170–172.
Greer K. R. 1957. Some osteological characters of known-age ranch minks. Journal of Mammalogy 38: 319–330.
Hagmeier E. 1961. Variation and relationships in North American marten. Canadian Field-Naturalist 75: 122–138.
Hays W. S. T. and Simberloff D. 2006. A morphometric trend linked to male sociality in the small Indian mongooseHerpestes javanicus in Hawaii. Acta Theriologica 51: 303–310.
Henry S. E. and Raphael M. G. 1989. Observations of copulation of free-ranging American marten. Northwestern Naturalist 70: 32–33.
Hewer H. R. 1964. The determination of age, sexual maturity, longevity and a life-table in the grey seal (Halichoerus grypus). Proceedings of the Zoological Society of London 142: 593–624.
Holmes T. and Powell R. A. 1994. Morphology, ecology, and the evolution of sexual dimorphism in North AmericanMartes. [In: Martens, sables, and fishers: biology and conservation. S. W. Buskirk, A. S. Harestad, M. G. Raphael and R. A. Powell, eds]. Cornell University Press, Ithaca, New York: 72–84.
Hosken D. J. and Stockley P. 2004. Sexual selection and genital evolution. Trends in Ecology and Evolution 19: 87–93.
Izor R. J. and de la Torre L. 1978. A new species of weasel (Mustela) from the highlands of Colombia, with comments on the evolution and distribution of South American weasels. Journal of Mammalogy 49: 92–102.
Jones I. L., Hunter F. M. and Fraser G. 2000. Patterns of variation in ornaments of crested aukletsAethia cristatella. Journal of Avian Biology 3:119–1277.
Kelly D. A. 2000. Anatomy of the baculum-corpus cavernosum interface in the Norway rat (Rattus norvegicus), and implications for force transfer during copulation. Journal of Morphology 244: 69–77.
Kinahan A., Bennett N., O’Riain M., Hart L. and Bateman P. 2007. Size matters: genital allometry in an African mole-rat (Family: Bathyergidae). Evolutionary Ecology 21: 201–213.
King C. M. and Powell R. A. 2006. The natural history of weasels and stoats: ecology, behavior, and management. Second ed. Oxford University Press, New York: 1–464.
Klevezal G. A., Mina A. V. and Krushinskaya N. L. 2005. Application of methods of multivariate statistical analysis for determining age of mammals (with special reference to the northern birch mouse,Sicista betulina, and the pine marten,Martes martes). Zoologicheskii Zhurnal 84: 1389–1401. [In Russian with English summary]
Kodric-Brown A., Sibly R. M. and Brown J. H. 2006. The allometry of ornaments and weapons. Proceedings of the National Academy of Sciences of the U.S.A. 103: 8733–8738.
Larivière S. and Ferguson S. H. 2002. On the evolution of the mammalian baculum: vaginal friction, prolonged intromission or induced ovulation? Mammal Review 32: 283–294.
Leach D. 1977. The descriptive and comparative postcranial osteology of marten (Martes americana Turton) and fisher (Martes pennanti Erxleben): the appendicular skeleton. Canadian Journal of Zoology 55: 199–214.
Lidicker W. Z. Jr and Yang A. 1986. Morphology of the penis in the taiga vole (Microtus xanthognathus). Journal of Mammalogy 67: 497–502.
Long C. A. 1968. An analysis of patterns of variation in some representative Mammalia. Part I. A review of estimates of variability in selected measurements. Transactions of the Kansas Academy of Science 71: 201–227.
Long C. A. 1969. Gross morphology of the penis in seven species of the Mustelidae. Mammalia 33: 145–160.
Long C. A. and Frank T. 1968. Morphometric variation and function in the baculum, with comments on correlation of parts. Journal of Mammalogy 49: 32–43.
Long C. A. and Jones C. J. 1966. Variation and frequency of occurrence of the baculum in a population of Mexican free-tailed bats. Southwestern Naturalist 11: 290–295.
Lönnberg E. 1911. Der Penisknochen zweifer seltener Carnivoren. Anatomischer Anzeiger 38: 230–232.
Lüpold S., McElligot A. G. and Hosken D. J. 2004. Bat genitalia: allometry, variation and good genes. Biological Journal of the Linnean Society 83: 497–507.
Maeda K. 1978. Baculum of the Japanese large noctule,Nyctalus lasiopoterus aviator Thomas 1911. Acta Anatomica Nipponica 53: 447–453. [In Japanese with English summary]
Manly B. F. J. 1986. Multivariate statistical methods: a primer. Chapman and Hall, London, England: 1–215.
Markley M. H. and Bassett C. F. 1942. Habits of captive marten. American Midland Naturalist 28: 604–616.
Marshall W. H. 1951. An age determination method for the pine marten. The Journal of Wildlife Management 15: 276–283.
Mead R. A. 1994. Reproduction inMartes. [In: Martens, sables, and fishers: biology and conservation. S. W. Buskirk, A. S. Harestad, M. G. Raphael and R. A. Powell, eds]. Cornell University Press, Ithaca, New York: 404–422.
Miller E. H. and Burton L. E. 2001. It’s all relative: allometry and variation in the baculum (os penis) of the harp sealPagophilus groenlandicus (Carnivora: Phocidae). Biological Journal of the Linnean Society 72: 345–355.
Miller E. H., Jones I. L. and Stenson G. B. 1999. Baculum and testes of the hooded seal (Cystophora cristata): growth and size-scaling and their relationships to sexual selection. Canadian Journal of Zoology 77: 470–479.
Miller E. H., Pitcher K. W. and Loughlin T. R. 2000. Bacular size, growth, and allometry in the largest extant otariid, the Steller sea lion (Eumetopias jubatus). Journal of Mammalogy 81: 134–144.
Miller E. H., Stewart A. R. J. and Stenson G. B. 1998. Bacular and testicular growth, allometry, and variation in the harp seal (Pagophilus groenlandicus). Journal of Mammalogy 79: 502–513.
Mohr E. 1963. Os penis und Os clitoridis der Pinnipedia. Zeitschrift für Säugetierkunde 28: 19–37.
Nagorsen D. W. 1994. Body weight variation among insular and mainland American martens. [In: Martens, sables, and fishers: biology and conservation. S. W. Buskirk, A. S. Harestad, M. G. Raphael and R. A. Powell, eds]. Cornell University Press, Ithaca, New York: 85–97.
Nagorsen D. W., Forsberg J. and Giannico G. R. 1988. An evaluation of canine radiographs for sexing and aging Pacific Coast martens. Wildlife Society Bulletin 16: 421–426.
Oosthuizen W. H. and Miller E. H. 2000. Bacular and testicular growth and allometry in the Cape fur sealArctocephalus p. pusillus (Otariidae). Marine Mammal Science 16: 124–140.
Patterson B. D. 1983. Baculum-body size relationships as evidence for a selective continuum on bacular morphology. Journal of Mammalogy 64: 496–499.
Patterson B. D. and Thaeler C. S. J. 1982. The mammalian baculum: hypotheses on the nature of bacular variability. Journal of Mammalogy 63: 1–15.
Petrides G. A. 1950. The determination of sex and age ratios in fur animals. American Midland Naturalist 43: 355–382.
Petrie M. 1992. Are all secondary display structures positively allometric and, if so, why? Animal Behaviour 43: 173–175.
Pocock R. I. 1918. The baculum or os penis of some genera of Mustelidae. Annals and Magazine of Natural History, Series 9(1): 307–312.
Poole K. G., Matson G. M., Strickland M. A., Magoun A. J., Graf R. P. and Dix L. M. 1994. Age and sex determination for American martens and fishers. [In: Martens, sables, and fishers: biology and conservation. S. W. Buskirk, A. S. Harestad, M. G. Raphael and R. A. Powell, eds]. Cornell University Press, Ithaca, New York: 204–223.
Powell R. A. 1993. The fisher: life history, ecology, and behavior, second ed. University of Minnesota Press, Minneapolis, Minnesota: 1–237.
Powell R. A., Buskirk S. W. and Zielinski W. J. 2003. Fisher and martenMartes pennanti andMartes americana. [In: Wild mammals of North America: biology, management, and conservation. Second ed. G. A. Feldhamer, B. C. Thompson and J. A. Chapman, eds]. The Johns Hopkins University Press, Baltimore, Maryland: 635–649.
Proulx G., Aubry K., Birks J., Buskirk S., Fortin C., Frost H., Krohn W., Mayo L., Monakhov V., Payer D., Saeki M., Santos-Reis M., Weir R. and Zielinski W. 2004. World Distribution and status of the genusMartes in 2000. [In: Martens and fishers (Martes) in human-altered environments: an international perspective. D. J. Harrison, A. K. Fuller and G. Proulx, eds]. Springer Science+ Business Media, New York: 21–76.
Ramm S. A. 2007. Sexual selection and genital evolution in mammals: a phylogenetic analysis of baculum length. The American Naturalist 169: 360–369.
Rausch R. A. and Pearson A. M. 1972. Notes on the wolverine in Alaska and the Yukon Territory. The Journal of Wildlife Management 36: 249–268.
Ruggiero L. F. and Henry S. E. 1993. Courtship and copulatory behavior ofMartes americana. Northwestern Naturalist 74: 18–22.
Ruth A. B. 1934. The os priapi: a study in bone development. Anatomical Record 60: 231–249.
Sachs B. D., Glater G. B. and O’Hanlon J. K. 2005. Morphology of the erect glans penis in rats under various gonadal hormone conditions. Anatomical Record 210: 45–52.
Sanderson G. C. 1961. Techniques for determining age of raccoons. Illinois Natural History Survey Division, Biological Notes 45: 1–16.
Sanvito S., Galimberti F. and Miller E. H. 2007a. Having a big nose: structure, ontogeny, and function of the elephant seal proboscis. Canadian Journal of Zoology 85: 207–220.
Sanvito S., Galimberti F. and Miller E. H. 2007b. Vocal signalling of male southern elephant seals is honest but imprecise. Animal Behaviour 73: 287–299.
Scheffer V. B. 1950. Growth of the testes and baculum in the fur seal,Callorhinus ursinus. Journal of Mammalogy 31: 384–394.
Scheffer V. B. and Kenyon K. W. 1963. Baculum size in pinnipeds. Zeitschrift für Säugetierkunde 28: 38–41.
Smirnov D. G. and Tsytsulina K. 2003. The ontogeny of the baculum inNyctalus noctula andVespertilio murinus (Chiroptera: Vespertilionidae). Acta Chiropterologica 5: 117–123.
Stone K. D. and Cook J. A. 2002. Molecular evolution of Holarctic martens (genusMartes, Mammalia: Carnivora: Mustelidae). Molecular Phylogenetics and Evolution 24: 169–179.
Stone K. D., Flynn R. W. and Cook J. A. 2002. Post-glacial colonization of northwestern North America by the forest-associated American marten (Martes americana, Mammalia: Carnivora: Mustelidae). Molecular Ecology 11: 2049–2063.
Strickland A. W. and Douglas C. W. 1982. Marten. [In: Wild furbearer management and conservation in North America. M. Novak, J. A. Baker, M. E. Obbard and B. Malloch, eds]. Ontario Trappers’ Association, Toronto: 530–546.
Strickland M. A., Douglas C. W., Novak M. and Hunziger N. P. 1987. MartenMartes americana. [In: Wild mammals of North America: biology, management, economics. J. A. Chapman and G. A. Feldhamer, eds]. Johns Hopkins University Press, Baltimore, Maryland: 599–612.
Tasikas D. E., Fairn E. R., Laurence S. and Schulte-Hostedde A. I. 2007. Baculum variation and allometry in the muskrat (Ondatra zibethicus): a case for sexual selection. Evolutionary Ecology. DOI 10.1007/s 10682-007-9216-2
Tatsuta H., Mizota K. and Akimoto A.-I. 2001. Allometric patterns of heads and genitalia in the stag beetleLucanus maculifemoratus (Coleoptera: Lucanidae). Annals of the Entomological Society of America 94: 462–466.
Uhl G. and Vollrath F. 2000. Extreme body size variability in the golden silk spider (Nephila edulis) does not extend to genitalia. Journal of Zoology, London 251: 7–14.
Van Valen L. 1978. The statistics of variation. Evolutionary Theory 4: 33–43.
Ventura J. 1993. Relative growth of sexual organs in males ofArvicola terrestris (Rodentia, Arvicolidae) from the Iberian Peninsula. Zeitschrift für Säugetierkunde 58: 124–126.
Wheeler D., Wong A. and Ribeiro J. M. C. 1993. Scaling of feeding and reproductive structures in the mosquitoAedes aegypti L. (Diptera: Culicidae). Journal of the Kansas Entomological Society 66: 121–124.
Whelton H. J. and Power S. B. 1993. The use of badger bacula as a method of age determination in a badger population infected with tuberculosis. Biology and Environment: Proceedings of the Royal Irish Academy 93B: 45–47.
Wright P. L. 1947. The sexual cycle of the male long-tailed weasel (Mustela frenata). Journal of Mammalogy 28: 343–352.
Wright P. L. and Coulter M. W. 1967. Reproduction and growth in Maine fishers. The Journal of Wildlife Management 31: 70–87.
Wright P. L. and Rausch R. 1955. Reproduction in the wolverine,Gulo gulo. Journal of Mammalogy 36: 346–355.
Yablokov A. V. 1974. Variability of mammals. Amerind Publishing Company, New Delhi, India: 1–350.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate editor was Andrzej Zalewski.
Rights and permissions
About this article
Cite this article
Miller, E.H., Nagorsen, D.W. Bacular variation and allometry in the western martenMartes caurina . Acta Theriol 53, 129–142 (2008). https://doi.org/10.1007/BF03194246
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03194246