Summary
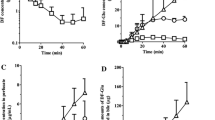
Single oral doses of14C-dexloxiglumide were rapidly and extensively absorbed in rats, and eliminated more slowly by females than by males. The respective half-lives were about 4.9 and 2.1h. Following single intravenous doses, dexloxiglumide was characterised as a drug having a low clearance (6.01 and about 1.96 ml/min/kg in males and females respectively), a moderate volume of distribution (Vss, 0.98 and about 1.1 L/kg in males and females respectively) and a high systemic availability. It was extensively bound to plasma proteins (97%). Dexloxiglumide is mainly cleared by the liver. Its renal clearance was minor. In only the liver and gastrointestinal tract, were concentrations of14C generally greater than those in plasma. Peak14C concentrations generally occurred at 1–2h in males and at 2–4h in females. Tissue14C concentrations then declined by severalfold during 24h although still present in most tissues at 24h but only in a few tissues (such as the liver and gastrointestinal tract) at 168h. Decline of14C was less rapid in the tissues of females than in those of males. Single intravenous or oral doses were mainly excreted in the faeces (87–92%), mostly during 24h and more slowly from females than from males. Urines contained less than 11% dose. Mean recoveries during 7 days when14C was not detectable in the carcass except in one female rat ranged between 93–101%. Biliary excretion of14C was prominent (84–91% dose during 24h) in the disposition of14C which was also subjected to facile enterohepatic circulation (74% dose). Metabolite profiles in plasma and selected tissues differed. In the former, unchanged dexloxiglumide was the major component whereas in the latter, a polar component was dominant. Urine, bile and faeces contained several14C-components amongst which unchanged dexloxiglumide was the most important (eg. up to 63% dose in bile). LC-MS/MS showed that dexloxiglumide was metabolised mainly by hydroxylation in the N-(3-methoxypropyl)pentyl sidechain and by O-demethylation followed by subsequent oxidation of the resulting alcohol to a carboxylic acid.
Similar content being viewed by others
References
Woodruff G.N. and Hughes J. (1991): Cholecystokinin antagonists. Ann. Rev. Pharmacol. Toxicol., 31, 469–501.
D’Amato M., Makovec F., Rovati L.C. (1999b): CCKA-receptors in gastrointestinal disorders. Drug Development: Molecular Targets for GI Diseases, edited by T.S. Gaginella and A. Guglietta (Totowa, NJ: Human Press) pp. 147–175.
Grider J.R. (1994): Role of cholecystokinin in the regulation of gastrointestinal motility. J. Nutrit., 124 (Suppl. 8), 1334S-1339S.
Revel L., Makovec F., Castaner J. (1999): Dexloxiglumide — CCK1 (CCKA) receptor antagonist treatment of irritable bowel syndrome. Drugs of the Future, 24, 725–728.
Scarpignato C., Kisfalvi I., D’Amato M., and Varga G. (1996): Effect of dexloxiglumide and spiroglumide, two new CCK-receptor antagonists, on gastric emptying and secretion in the rat: evaluation of their receptor selectivity in vivo. Alimen. Pharmacol. Ther., 10, 411–419.
Fioramonti J., D’Amato M., Rovati L., Buéno L. (1996): Effects of dexloxiglumide on gastric emptying delayed by colonic distension in dogs: Neurogastroenterol. Motil., 8, 172.
Rouzade M.L., Fioramonti J., D’Amato M., Rovati L., Buéno L. (1996): Inhibitory action of dexloxiglumide on transient lower oesophageal sphincter relaxation in dogs. Neurogastroenterol. Motil., 8, 188.
Meier R., Beglinger C., Giacovelli G., D’Amato M. (1997): Effect of the CCK-A receptor antagonist dexloxiglumide on postprandial gallbladder emptying and colonic transist time in healthy volunteers, Gastroenterol., 112, A788.
D’Amato M., Whorwell P.J., Thompson D.G., Spiller R.C., Giacovelli G., Rovati L.C., (1999a): The efficacy and safety of the CCKA-receptor antagonist dexloxiglumide in IBS. Gut, 45 (Suppl. V), A258.
D’Amato M., Whorwell P.J., Thompson D.G., Spiller R.C., Giacovelli G., Griffin P.H., Schneier H. and Rovati L.C. (2001): CCK1-receptor antagonist dexloxiglumide is effective and safe in female patients with constipation predominant irritable bowel syndrome. Amer. J. Gastroenterol., 96, S317.
Feinle C., Meier O., D’Amato M. (1999): CCK-A Receptor blockade improves dyspeptic symptoms due to duodenal lipid and gastric distension in functional dyspepsia. Gastroenterol., 116, (Suppl.), A992.
Webber C., Roth A., Persiani S., Peard A.J., Makovec A., John B.A., Holding J.D., Hackett A.M., D’Amato M., Cybulski Z.R. and Chasseaud L.F. (2002): Pharmacokinetics and metabolism of the cholecystokinin antagonist dexloxiglumide in male human subjects. Drug Metab. Rev., 34 (Suppl. 1), 79.
Webber C., Roth A., Persiani S., Peard A.J., Makovec A., Kapil R.P., John B.A., Holding J.D., D’Amato M., Cybulski Z.R., Chasseaud L.F. and Rovati L.C. (2003): Pharmacokinetics and metabolism of the cholecystokinin antagonist dexloxiglumide in male human subjects. Xenobiotica, 33, 625–641.
Ullberg S. and Larsson B. (1981): Whole-body autoradiography. In Progress in Drug Metabolism, edited by J.W. Bridges and L.F. Chasseaud (Chichester: Wiley), vol. 6, 249–289.
Davies B. and Morris T. (1993): Physiological parameters in laboratory animals and humans. Pharm. Res., 10, 1093–1095.
Waxman D.J., Dannan G.A. and Guengerich F.P. (1985): Regulation of rat hepatic cytochrome P-450: age dependent expression, hormonal imprinting, and xenobiotic inducibility of sex-specific isozymes. Biochemistry, 24, 4409–4417.
Skett P., (1987): Hormonal regulation and sex differences of xenobiotic metabolism. In Progress in Drug Metabolism, edited by J.W. Bridges, L.F. Chasseaud and G.G. Gibson (London: Taylor and Francis), vol. 10, 85–140.
Hucker H.B., Kwan K.C., Duggan D.E. (1980): Pharmacokinetics and metabolism of non-steroidal anti-inflammatory agents. In Progress in Drug Metabolism, edited by J.W. Bridges and L.F. Chasseaud, (Chichester: Wiley), vol. 5, 165–253.
Hirom P.C., Idle J.R. and Millburn P. (1977): Comparative aspects of the biosynthesis and excretion of xenobiotic conjugates by non-primate mammals. In Drug Metabolism — From Microbe to Man, edited by D.V. Parke and R.L. Smith, (London: Taylor and Francis), 299–329.
Okuda S. and Matsuzawa T. (1970): Absorption, distribution, excretion and metabolism of D,L-4-Benzamide-N,N-di-n-propyl-glutaramic acid (proglumide) in rats. J. Takeda Res. Lab., 29, 93–101.
Gibson G.G. and Skett P. (1986): Introduction to Drug Metabolism, (London: Chapman and Hall).
Chasseaud L.F. and Hawkins D.R. (1990): Biotransformation of drugs. In Encyclopedia of Pharmaceutical Technology, edited by J. Swarbrick and J.C. Boylan, (New York: Marcel Dekker), 129–157.
Fieser L.F. and Fieser M. (1956): Organic Chemistry, (New York: Reinhold Publishing Corporation).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Webber, C., Stokes, C.A., Persiani, S. et al. Absorption, distribution, metabolism and excretion of the cholecystokinin-1 antagonist dexloxiglumide in the rat. Eur. J. Drug Metab. Pharmacokinet. 28, 201–212 (2003). https://doi.org/10.1007/BF03190486
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190486