Summary
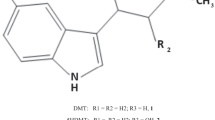
para-Hydroxytranylcypromine (p-OHTCP) has recently been unequivocally identified in our laboratory as a metabolite of the antidepressant tranylcypromine (TCP). In the study reported here, we have determined brain and heart levels ofp-OHTCP in the rat after intraperitoneal administration of a 0.1 mmol/kg dose of TCP or N-(2-cyanoethyl)tranylcypromine (CE-TCP). The animals were killed at 5, 15, 30, 60, 120 or 240 min after drug administration and the tissues (brain and heart) rapidly dissected out. The tissues were frozen in isopentane on solid carbon dioxide and stored at −20°C until time of analysis. Tissue levels ofp-OHTCP, TCP and CE-TCP were determined after aqueous pentafluorobenzoylation by conducting analyses with a gas-liquid chromatograph equipped with a fused silica (SE-54) capillary column and an electron-capture detector. Our results show that substantial concentrations ofp-OHTCP were present in both brain and heart after TCP and CE-TCP administration. Higher levels ofp-OHTCP were present in the brain than in the heart after TCP treatment, but this situation was reversed with the CE-TCP-treated rats. Sincep-OHTCP has been shown to retain some MAO-inhibiting properties and to have effects on uptake of catecholamines and serotonin it could therefore contribute to the pharmacological profile of TCP.
Similar content being viewed by others
References
Martindale: The Extra Pharmacopoeia, 28th ed., (1982): (eds., Reynolds J.E.F. and Prasad A.B.), pp. 131–132. Pharmaceutical Press, London.
Fuentes J.A., Oleshansky M.A. and Neff N.H. (1976): Comparison of the apparent antidepressant activity of (-) and (+) tranylcypromine in an animal model.Biochem. Pharmacol. 25, 801–804.
Calverley D.G., Baker G.B., Coutts R.T. and Dewhurst W.G. (1981): A method for measurement of tranylcypromine in rat brain regions using gas chromatography with electron-capture detection.Biochem. Pharmacol. 30, 861–867.
Nazarali A.J., Baker G.B., Coutts R.T. and Wong T.F.J. (1987): N-(2-cyanoethyl)tranylcypromine, a potential prodrug of tranylcypromine: its disposition and interaction with catecholamine neurotransmitters in brain.Pharm. Res. 4, 16–20.
Beckett A.H., Shenoy E.V.B, and Salmon J.A. (1972): The influence of replacement of the N-ethyl group by the cyanoethyl group on the absorption, distribution and metabolism of (+)-ethylamphetamine in man.J. Pharm. Pharmacol.24, 194–202.
Tognoni G., Morselli P.L. and Garattini S. (1972): Amphetamine concentrations in rat brain and human urine after fenproporex administration. Eur. J. Pharmacol.20, 125–126.
Nazarali A.J., Baker G.B., Coutts R.T. and Pasutto F.M. (1983): Amphetamine in rat brain after intraperitoneal injection of N-alkylated analogues.Prog. Neuropsychopharmacol. & Biol. Psychiat..7, 813–816.
Coutts R.T., Nazarali A.J., Baker G.B. and Pasutto F.M. (1986): Metabolism and disposition of N-(2-cyanoethyl) amphetamine (fenproporex) and amphetamine: study in the rat brain.Can. J. Physiol. Pharmacol. 64, 724–728.
Warembourg H. and Jaillard J. (1968): Expérimentation clinique due fenproporex dans le traitment des obésités.Lille Med 13, Suppl. 273.
Hertel G. and Fallot-Burghardt W. (1978): Treatment of obese female patients with fenproporex within the framework of gynecologic practice.Fortschr. Med 96, 2380–2382.
Martindale: The Extra Pharmacopoeia, (1982): 28th ed., (eds., Reynolds J.E.F. and Prasad A.B.), pp. 15–70. Pharmaceutical Press, London.
Baker G.B., Hampson D.R., Coutts R.T. Micetich R.G., Hall T.W. and Rao T.S. (1986): Detection and quantitation of a ring hydroxylated metabolite of the antidepressant drug tranylcypromine.J. Neural. Transm., 65, 233–243.
Winer B.J. (1971): Statistical Principles in Experimental Design. Second Edition, pp. 1–907, McGraw-Hill Book Company, N.Y.
Gibaldi M. and Perrier D. (1982): inPharmacokinetics, Second Edition, Revised and Expanded, vol. 15, pp. 1–494. Marcel Dekker, Inc., New York.
Coutts R.T. and Dawson G.W. (1977): Urinary excretion of phenolic metabolites of N-(n-propyl)amphetamine in man.Res. Commun. Chem. Pathol. Pharmacol. 17, 349–352.
Author information
Authors and Affiliations
Additional information
Funding was provided by the Provincial Mental Health Advisory Council (PMHAC) and by the Alberta Heritage Foundation for Medical Research.
Rights and permissions
About this article
Cite this article
Nazarali, A.J., Baker, G.B., Coutts, R.T. et al. Para-hydroxytranylcypromine: presence in rat brain and heart following administration of tranylcypromine and an N-cyanoethyl analogue. European Journal of Drug Metabolism and Pharmacokinetics 12, 207–214 (1987). https://doi.org/10.1007/BF03189899
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03189899