Abstract
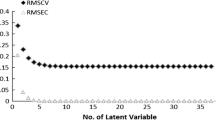
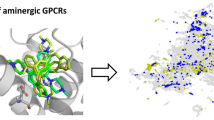
The study of three-dimensional quantitative structure-activity relationship (3D-QSAR) of DDPH and its derivatives that have been known with their activity parameters has been developed using the comparative molecular field analysis (CoMFA) method. Here, (+)-DDPH crystal structure was selected as the active conformation model and comparisons between the influences of different charge calculation methods and grid setup were conducted. The coefficients of cross-validation (q 2) and regression (r 2) are 0.481 and 0.997, respectively. The standard error (SE) is 0.102. The research result suggests that the steric field makes more contributions to the activity than the electrostatic field. This model can help us not only in improving our understanding of the receptor-ligand interactions, but also in predicting the activity of derivatives and designing new compounds with better potency.
Similar content being viewed by others
References
Xia, L., Ni, P. Z., Qian, J. Q. et al., Preparation of phenoxyalkylamine compounds as antihypertensive agents, China Patent, CN 1070623.1
Cramer, R. D., Patterson, D. E., Bunce, J. D., Comparative molecular field analysis (CoMFA) (I)—Effect of shape on binding of steroids to carrier proteins, J. Am. Chem. Soc., 1988, 110(18): 59.
Beuerle, G., Kovar, K. A., Schulze-Alexandru, M. et al., Three-dimensional quantitive structure-activity relationship of hallucinogenic phenylalkanime and tryptamine derivatives studies using comparative molecular field analysis (CoMFA), Quant. Struct-Act. Relat., 1997, 16(6): 447.
Matter, H., Schwab, W., Barbier, D. et al., Quantitative structure-activity relationship of human neutropil collagenase (MMP-8) inhibitors using comparative molecular field analysis and X-ray structure analysis, J. Med. Chem., 1999, 42 (11): 1908.
Liu, J., Li, Z. M., Wang, X. et al., Comparative molecular field analysis (CoMFA) of new herbicial sulfonylurea compounds, Sci. in China, Ser. B, 1998, 48(1): 50.
Song, W., Zhang, Y. J., Xia, L. et al., Effects of some mexiletine derivatives on α1-adrenoceptors, Acta Pharmaceutica Sinica (in Chinese), 1998, 33(2): 102.
Xu, J. Y., Xia, L., Ni, P. Z. et al., Synthesis and cardiovascular activity of some benzylethylamine derivatives, Journal of China Pharmaceutical University (in Chinese), 1992, 23(4): 203.
Ni, P. Z., Sun, H. B., Pen, J. H. et al., Resolution of (±)DDPH and α1-adrenoceptor antagonsign activities of both (±) and (-) DDPH, Journal of China Pharmaceutical University (in Chinese), 1996, 27(8): 462
Song, S. Y., Lin, X. Y., Xia, L. et al., Crystal structure of new drug DDPH and absolute configuration of (+)DDPH, Chinese Journal of Structural Chemistry (in Chinese), 1997, 16(2): 102.
Wang, R. X., Liu, G., Lai, L. H. et al., Role of compound orientation in CoMFA studies, Acta Physicochimica Sinca (in Chinese), 1998, 14(1): 1.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fang, H., Lu, J., Xia, L. et al. 3D-QSAR study on phenoxyalkylamine compounds of α1-adrenoceptor antagonist. Chin.Sci.Bull. 46, 303–306 (2001). https://doi.org/10.1007/BF03187190
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03187190