Abstract
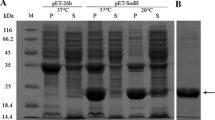
A MoFe protein (ΔnifE Avl) with a purity of ∼80% was purified from a nifE-deleted mutant ofAzotobacter vinelandii DJ35. Compared with MoFe protein purified from wild-type strain OP (OP Av1), ΔnifE Av1 had the same subunits composition, and had immune reaction with antibody to OP Av1, but its relative mobility in anaerobic native polyacrylamide gel electrophoresis (PAGE) was a little larger than that of OP Av1. Metal analysis showed that Mo and Fe contents of ΔnifE Av1 both apparently decreased. When complemented with OP Fe protein, ΔnifE Av1 had no C2H2-reduction activity, but it could bein vitro activated by FeMoco extracted from OP Av1. The circular dichroism (CD) spectrum of ΔnifE Av1 at ∼450 nm was similar to that of OP Av1, while the EPR signal at g∼3.7 was absolutely silent, and the signal intensities at g∼=4.3 and 2.0 decreased by 75% and 50%, respectively. The results indicated that ΔnifE Av1 purified from DJ35 was a FeMoco-deficient but P-cluster-containing MoFe protein.
Similar content being viewed by others
References
Howard, J. B., Rees, D. C., Structural basis of biological nitrogen fixation, Chem. Rev., 1996, 96: 2965–2982.
Kim, J., Rees, D. C., Structural models for the metal centers in the nitrogenase molybdenum-iron protein, Science, 1992, 257: 1677–1682.
Smith, B. E., Eady, R. R., Metalloclusters of the nitrogenases, Eur. J. Biochem., 1992, 205: 1–15.
Burgess, B. K., The iron-molybdenum cofactor of nitrogenase, Chem. Rev., 1990, 90: 1377–1406.
Tal, S., Chun, T. W., Gavini, N. et al., The ΔniB (or ΔnifE FeMo cofactor-deficient MoFe protein is different from the ΔnifH protein, J. Biol. Chem., 1991, 266: 10654–10657.
Gavini, N., Ma, L., Watt, G. et al., Purification and characterization of a FeMo cofactor-deficient MoFe protein, Biochemistry, 1994, 33: 11842–11849.
Ribbe, M. W., Hu, Y., Guo, M. et al., The FeMo-co-deficient MoFe protein produced by anifH deletion strain ofAzotobacter vinelandii shows unusual P-cluster features, J. Biol. Chem., 2002, 277: 23469–23476.
Schmid, B., Ribbe, M. W., Einsle, O. et al., Structure of a cofactor-deficient nitrogenase MoFe protein, Science, 2002, 296: 352–356.
Allen, R. M., Chatterjee, R., Ludden, P. W. et al., Incorporation of iron and sulfur from NifB cofactor into the iron-molybdenum cofactor of nitrogenase, J. Biol. Chem., 1995, 270: 26890–26896.
Roll, J. T., Shah, V. K., Dean, D. R. et al., Charateristics of NIFNE inAzotobacter vinelandii strains, J. Biol. Chem., 1995, 270: 4432–4437.
Goodwin, P. J., Agar, G. N., Roll, J. T. et al., TheAzotobacter vinelandii NifEN complex contains two identical [4Fe-4S] clusters, Biochemistry, 1998, 37: 10420–10428.
Suh, M. H., Pulakat, L., Gavini, N., Functional expression of the FeMo-cofactor-specific biosynthetic genesnifEN as a NifE-N fusion-protein synthesizing unit inAzotobacter vinelandii, Biochem. Biophys. Res. Commun., 2002, 299: 233–240.
Hu, Y., Fay, A. W., Ribbe, M. W., Identification of a nitrogenase FeMo cofactor precursor on NifEN complex, Proc. Natl. Acad. Sci. USA, 2005, 102: 3236–3241.
Siemann, S., Schneider, K., Behrens, K. et al., FeMo cofactor biosynthesis in a nifE− mutant ofRhodobacter capsulatus, Eur. J. Biochem., 2001, 268: 1940–1952.
Zhao, J. F., Zhao, Y., Wang, Z. P. et al., Purification and activationin vitro of MoFe protein from anifE deleted mutant strain ofAzotobacter vinelandii, Acta Bot. Sin., 2003, 45: 815–819.
Zhao, Y., Zhao, J. F., Lü, Y. B. et al., Crystallization of nitrogenase MoFe protein from a mutantnifE deleted strain ofAzotobacter vinelandii, Acta Bot. Sin., 2003, 45: 427–431.
Brigle, K. E., Weiss, M. C., Newton, W. E. et al., Products of the iron-molybdenum cofactor-specific biosynthetic genes,nifE andnifN, are structurally homologous to the products of nitrogenase molybdemum-iron protein genes,nifD andnifK, J. Bacteriol., 1987, 169: 1547–1553.
Strandberg, G. W., Wilson, P. W., Formation of the nitrogen-fixing enzyme system inAzotobacter vinelandii, Can. J. Microbiol., 1968, 14: 25–31.
Burgess, B. K., Jacobs, D. B., Stiefel, E. I., Large scale purification of high activityAzotobacter vinelandii nitrogenase, Biochim. Biophys. Acta, 1980, 614: 196–209.
Ornstein, L., Disc electrophoresis. I. Background and theory, Ann. N. Y. Acad. Sci., 1964, 121: 321–349.
Kuo, C. F., Fridovich, I., A stain for iron-containing proteins sensitive to nanogram levels of iron, Anal. Biochem., 1988, 170: 183–185.
Luo, A. L., Wang, J. W., Li, J. G., A simple method for protein blotting and immunoassay, Chinese Bull. Bot., 1995, 12: 63–64.
Laemmli, U. K., Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature, 1970, 227: 680–685.
Blanchard, C. Z., Hales, B. J., Isolation of two forms of nitrogenase VFe protein fromAzotobacter vinelandii, Biochemistry, 1996, 35: 472–478.
Robinson, A. C., Chun, T. W., Li, J. G. et al., Iron-molybdenum cofactor insertion into the apo-MoFe protein of nitrogenase involves the iron protein-MgATP complex, J. Biol. Chem., 1989, 264: 10088–10095.
McLean, P. A., Wink, D. A., Chapman, S. K. et al., A new method for extraction of iron-molybdenum cofactor (FeMo-co) from nitrogenase adsorbed to DEAE-cellulose. 1. Effects of anions, cations, and preextraction treatments, Biochemistry, 1989, 28: 9402–9406.
Wink, D. A., McLean, P. A., Hickman, A. B. et al., A new method for extraction of iron-molybdenum cofactor (FeMo-co) from nitrogenase adsorbed to DEAE-cellulose. 2. Solubilization of FeMo-co in a wide range of organic solvents, Biochemistry, 1989, 28: 9407–9412.
Rangaraj, P., Ryle, M. J., Lanzilotta, W. N. et al.,In vitro biosynthesis of iron-molybdenum cofactor and maturation of the nif-encoded apodinitrogenase. Effect of substitution for NifH with site-specifically altered forms of NifH, J. Biol. Chem., 1999, 274: 19778–19784.
Christiansen, J., Goodwin, P. J., Lanzilotta, W. N. et al., Catalytic and biophysical properties of a nitrogenase Apo-MoFe protein produced by a nifB-deletion mutant ofAzotobacter vinelandii, Biochemistry, 1998, 37: 12611–12623.
Hawkes, T. R., Smith, B. E., Purification and characterization of the inactive MoFe protein (NifB-Kp1) of the nitrogenase fromnifB mutants ofKlebsiella pneumoniae, Biochem. J., 1983, 209: 43–50.
Hawkes, T. R., Smith, B. E., The inactive MoFe protein (NifB− Kpl) of the nitrogenase fromnifB mutantsof Klebsiella pneumoniae: its interaction with FeMo-cofactor and the properties of the active MoFe protein formed, Biochem. J., 1984, 223: 783–792.
Hu, Y., Fay, A. W., Dos Santos, P. C. et al., Characterization ofAzotobacter vinelandii nifZ deletion strains, J. Biol. Chem., 2004, 279: 54963–54971.
Stephens, P. J., McKenna, C. E., McKenna, M. C. et al., Circular dichroism and magnetic circular dichroism of reduced molybdenum-iron ofAzotobacter vinelandii nitrogenase—Biochemstry, 1981, 20: 2857–2864.
Huang, J. F., Luo, A. L., Xie, X. M. et al., Study on the circular dichroism spectroscopy of the disassembly and assembly of metal clusters in molybdenum-iron protein, Acta Microbiol. Sin., 1991, 31: 232–239.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zhao, Y., Bian, S., Zhang, C. et al. Characterization of a FeMo cofactor-deficient MoFe protein from anifE-deleted strain (DJ35) ofAzotobacter vinelandii . Chin.Sci.Bull. 50, 2305–2310 (2005). https://doi.org/10.1007/BF03183740
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03183740