Abstract
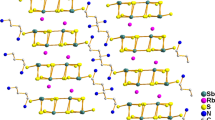
The Rb(NTO) · H2O crystal has been synthesized by reaction of 3-nitro-1,2,4-triazole-5-one (NTO) with Rb2CO3 in aqueous solution. Its crystal structure has been determined. The crystal belongs to monoclinic system. Crystal structure data: space group P211n; a=0.633 0(1),b = 0.824 1(2),c =1.296 4(3) nm; β =97.90(1)°;V= 0.669 9 (2) nm3,Z = 4,D c = 2.306 g/cm3, μ = 7.365 nm−1, F(000) = 448. An eight coordinated compound is formed between Rb+ with oxygen atoms and nitrogen atoms. A layer structure is formed by coordination bonds and hydrogen bonds. The thermal decomposition mechanism of this coordination compound is discussed.
Similar content being viewed by others
References
Rethgery, E. F., Audette, R. C., Wedlich, D. C., The study of the thermal decomposition of 3-nitro-1,2,4-triazol-5-one (NTO) by DSC, TG-AMS and ARC, Thermochim. Acta, 1991(2): 235.
Spear, R. J., Louey, C. N., Wolfson, M. G. et al., A Preliminary Assessment of 3-Nitro-1,2,4-Triazol-5-one (NTO) as an Insensitive High Explosive, MRL-Tb-89-18, Maribyrnong, Vic: Materials Research Laboratory, 1989.
Li Jiarong, Chen Boren, Crystal structure of ammonium 3-nitro-1,2,4-triazol-5-onate, Propellants Explosives Pyrotechnics, 1991, 16: 145.
Song Jirong, Hu Rongzu, Li Fuping et al., Preparation, molecular structure and thermodynamical properties of [Sr(NTO)2− (H2O)4]2 · 2H2O, Acta Chimica Simca (in Chinese), 2000, 58(2): 222.
Zhang Tonglai, Hu Rongzu, Li Fuping et al., Preparation, crystal structure and thermal decomposition mechanism of [Cu(NTO)2(H2O)] · 2H2O, Chinese Science Bulletin (in Chinese), 1993, 38(6): 523.
Song Jirong, Chen Zhaoxu, Xiao Heming et al., Preparation, crystal structure and quantum chemical investigation of [Li(NTO)(H2O)], Chinese Science Bulletin 1999, 44(3): 214.
Hu Rongzu, Song Jirong, Li Fuping, Preparation, crystal structure, the decomposition mechanism and thermodynamical properties of (Dy(NTO)2 · (H2O)6] · NTO · H2O, Thermochim. Acta, 1997, 299: 87.
Geilonann, W., Gebauhr, W., Zur Fallung der alkalimetalle als tetraphenylborverbindungen, Z. Anal. Chem., 1953, 139: 161.
Song Jirong, Study on Metal Coordination Compounds of NTO (in Chinese), Beijing: Chemical Industry Publishing Company, 1998, 1–3.
Prabbakaran, K. V., Naidu, S. R., XRD spectroscope and thermal analysis studies on NTO, Thermochim. Acta, 1994, 241: 199.
Song Jirong, Chen Zhaoxu, Xiao Heming et al., Preparation, crystal structure and quantum chemical investigation of [Yb(NTO)3 (H2O)4], Acta Chimica Simca (in Chinese), 1998, 56 (3): 270.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hu, M., Gao, S., Liu, Z. et al. Synthesis, crystal structure and thermal behavior of Rb(NTO) · H2O. Chin.Sci.Bull. 46, 45–48 (2001). https://doi.org/10.1007/BF03183207
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03183207