Abstract
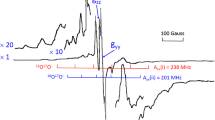
The electron paramagnetic resonance (EPR) signals of photoexcited quartet (Q1) states for zinc(II) tetra-tert-butyl-phthalocyanine (ZnPc) ligated by 3- and 4-(N-nitronyl-nitroxide) pyridine radicals (3-NOPy, 4-NOPy) were observed in toluene solution at room temperature by means of X-band (9.4 GHz) time-resolved EPR (TREPR) spectroscopy. Theg values of Q1 in the ZnPc-3-NOPy and ZnPc-4-NOPy complexes were found to beg=2.0025 andg=2.0036, respectively. The obtainedg value (2.0036) for ZnPc-4-NOPy is in good agreement with the value (g=2.0037) of the Q1 state calculated under the strong-exchange limit. Theg value (2.0025) is just an average of the Q1 and D1 (g=2.0013) states for ZnPc-3-NOPy. Theg value of Q1 for zinc(II) meso-tetraphenylporphine (ZnTPP) ligated by 3-NOPy showed a slight shift (g=2.0027) at X-band and no shift (g=2.0031) at W-band from the calculatedg value (g=2.0031) (J. Fujisawa, Y. Iwasaki, Y. Ohba, S. Yamauchi, K. Koga, S. Karasawa, M. Fuhs, K. Möbius, S. Weber, Appl. Magn. Reson. 21, 483–493, 2001). These changes in theg value were found to originate from an averaging of the TREPR spectra over the Q1 and photoexcited doublet (D1) states via a fast intersystem crossing (ISC) process. The ISC rates between these two states were estimated by means of numerical calculations with the modified Bloch equations as 1.2·108 and 6·107 s−1 for the ZnTPP-3-NOPy complex at the X- and W-bands, respectively. The lower limit of the ISC rate was obtained as 109s−1 for the ZnPc-3-NOPy complex and the higher limit was found to be 3.1·108 s−1 for the ZnPc-4-NOPy complex.
Similar content being viewed by others
References
Steiner U.E., Ulrich T.: Chem. Rev.89, 51 (1989)
Iwasaki Y., Maeda K., Murai H.: J. Phys. Chem. A105, 2961 (2001)
Mori Y., Sakaguchi Y., Hayashi H.: J. Phys. Chem. A106, 4453 (2002)
Blättler C., Jent F., Paul H.: Chem. Phys. Lett.166, 375 (1990)
Kawai A., Okutsu T., Obi K.: J. Phys. Chem.95, 9130 (1991)
Kobori Y., Takeda K., Tsuji K., Kawai A., Obi K.: J. Phys. Chem. A102, 5160 (1998)
Saiful I.M., Fujisawa J., Kobayashi N., Ohba Y., Yamauchi S.: Bull. Chem. Soc. Jpn.72, 661 (1999)
Blank A., Levanon H.: J. Phys. Chem. A105, 4799 (2001)
Shushin A.I.: Chem. Phys. Lett.208, 173 (1993)
Atkins P.W., Evans G.T.: Mol. Phys.29, 921 (1975)
Fujisawa J., Iwasaki Y., Ohba Y., Yamauchi S., Koga K., Karasawa S., Fuhs M., Möbius K., Weber S.: Appl. Magn. Reson.21, 483 (2001)
Fujisawa J., Ishii K., Ohba Y., Yamauchi S., Fuhs M., Möbius K.: J. Phys Chem. A103, 213 (1999)
Fujisawa J., Ishii K., Ohba Y., Yamauchi S., Fuhs M., Möbius K.: J. Phys. Chem. A101, 5869 (1997)
Mizuochi N., Ohba Y., Yamauchi S.: J. Phys Chem. A101, 5996 (1997)
Mizuochi N., Ohba Y., Yamauchi S.: J. Chem. Phys.111, 3479 (1999)
Ishii K., Kobayashi N.: Coord. Chem. Rev.198, 231 (2000)
Ishii K., Ishizaki T., Kobayashi N.: J. Chem. Soc. Dalton Trans.2001, 3227.
Ishii K., Takeuchi S., Kobayashi N.: J. Phys. Chem. A105, 6794 (2001)
Corvaja C., Maggini M., Prato M., Scrrano G., Venzin M.: J. Am. Chem. Soc.117, 8857 (1995)
Corvaja C., Maggini M., Ruzzi M., Scorrano G., Toffoletti A.: Appl. Magn. Reson.12, 477 (1997)
Kitano M., Ishimura Y., Inoue K., Koga N., Iwamura H.: Inorg. Chem.33, 6012 (1994)
Goudsmit G.H., Paul H.: Chem. Phys. Lett.208, 73 (1993)
Yamauchi S., Takahashi A., Iwasaki Y., Unno M., Ohba Y., Higuchi, J., Blank A., Levanon H.: J. Phys. Chem. A (in press)
Pool C.P., Farach H.A.: Relaxation in Magnetic Resonance, pp. 69–75. New York: Academic Press 1971.
Weissberger A.: Organic Solvents, p. 31. New York: Wiley 1970
Bencini A., Gatteschi D.: Electron Paramagnetic Resonance of Exchange Coupled Systems, pp. 49–57. Berlin: Springer 1990.
Akiyama K.: Phthalocyanine (Shirai H., Kobayashi N., eds.), p. 154. Tokyo: IPC 1997.
Seth J., Bocian D.F.: J. Am. Chem. Soc.116, 143 (1994)
Ishii K., Fujisawa J., Adachi A., Yamauchi S., Kobayashi N.: J. Am. Chem. Soc.120, 3152 (1998)
Carrington A., McLachlan A.D.: Introduction to Magnetic Resonance, pp. 204–208. New York: Harper & Row 1967.
Hore P.J., McLauchlan K.A.: Mol. Phys.42, 533 (1981)
Jäger M., Norris J.R. Jr: J. Phys. Chem. A106, 3659 (2002)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iwasaki, Y., Katano, K., Ohba, Y. et al. On theg-value shift and intersystem crossing in photo-excited radical-triplet systems. Appl. Magn. Reson. 23, 377–391 (2003). https://doi.org/10.1007/BF03166628
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF03166628