Abstract
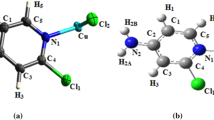
The CuL2X2 (L = 7-chlor-l,3-dihydro-3-hydroxi-5-phenyl-2H-l,4-benzodiazepin-2-one, also known as oxazepam, X = Cl, Br) complexes were prepared and investigated by ESR (electron spin resonance) method. Powder ESR spectra of CuL2X2 compounds at room temperature show the presence of monomeric species having an axial symmetry with a small rhombic distortion. In case of compounds with Cl there is a superposition of two nonequivalent mononuclear species, one with a\(d_{x^2 - y^2 } \) ground state and another with a\(d_{z^2 } \) ground state. In pyridine (Py) and dimethylformamide (DMF) solutions the monomeric species prevail. Two different monomeric species, one with pseudotetrahedral (Td) and the other with elongated tetrahedral-octahedral symmetry, were evidenced in DMF solutions adsorbed on NaY zeolite. In Py-DMF solutions two monomeric species were also identified. Dimeric species appear in DMF and Py solutions adsorbed on NaY zeolite through the coordination of two Cu(II) ions at the same keto-oxygen from one oxazepam molecule.
Similar content being viewed by others
References
Preti C., Tosi G.: J. Coord. Chem.6, 81 (1976)
Preti C., Tosi G.: J. Coord. Chem.8, 223 (1979)
Preti C., Tosi G.: Transition Met. Chem.3, 246 (1978)
Minghetti G., Ganadu M.L., Foddai C., Cinellu M.A., Demartin F., Manassero M.: Inorg. Chim. Acta86, 93 (1984)
Cinellu M.A., Ganadu M.L., Minghetti G., Cariati F., Demartin F., Manassero M.: Inorg. Chim. Acta143, 197 (1988)
Lu Z., Duan C., Tian Y.X., Huang X.: Inorg. Chem.35, 2253 (1996)
Yokoi H., Addison A.W.: Inorg. Chem.16, 1341 (1977)
Mabbs F.E., Collinson N.I. in: Electron Paramagnetic Resonance of Transition Metal Compounds. Amsterdam: Elsevier 1992.
Bencini A., Gatteschi D. in: Transition Metal Chemistry, vol. 8, p. 1. Berlin: Springer 1982.
Sakaguchi, Addison A.W.: J. Chem. Soc. Dalton Trans.8, 585 (1978)
Thomas K.J.J., Tharmaraj, Chandrasekhar: Inorg. Chem.33, 5382 (1994)
Batra G., Mathur P.: Inorg. Chem.31, 1575 (1992)
Ottaviani M.F.: Colloids Surfaces12, 305 (1984)
Cozar O., David L., Chis V., Cosma C., Znamirovschi V., Damian G., Bratu I., Bora Gh.: Appl. Magn. Reson.8, 235 (1995)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Forizs, E., David, L., Cozar, O. et al. ESR study of some Cu(II)-oxazepam complexes. Appl. Magn. Reson. 16, 499–506 (1999). https://doi.org/10.1007/BF03161946
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF03161946