Abstract
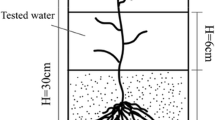
Oxides of Fe were dominant in composite sediment samples from the upper region of simulated compost wetlands exposed to acidic mine water, accounting for nearly half of substrate iron among the three sampling stations. In the middle and lower wetland regions, however, 65% of all Fe was retained as oxidizable Fe. Inferential evidence suggests that sulfate reduction was likely to be a critical process in Fe retention over at least half of the wetland length. However, overall, oxides of Fe (including amorphous and crystalline oxides) constituted the dominant phase of Fe in the wetland. The addition of a supplemental carbon source significantly stimulated both the formation of oxidizable Fe and reducible Fe in the middle and lower wetland regions, but not near the inlet. The use of effective carbon supplements is therefore recommended in field tests at acidic sites. Over the short term, the presence or species of vegetation had no effect on the partitioning of Fe phases.
Similar content being viewed by others
Literature Cited
Brodie, G. A., D. A. Hammer, and D. A. Tomljanovich. 1988. Constructed wetlands for acid drainage control in the Tennessee Valley. p. 325–331.In Mine Drainage and Surface Mine Reclamation. Vol. 1: Mine Water and Mine Waste. Bureau of Mines Information Circular 9183, Pittsburgh, PA, USA.
Brodie, G. A. 1993. Staged, aerobic constructed wetlands to treat acid drainage: case history of Fabius Impoundment 1 and overview of the Tennessee Valley Authority’s program. p. 157–165.In G. A. Moshiri (ed.) Constructed Wetlands for Water Quality Improvement. Lewis Publishers, Boca Raton, FL, USA.
Dvorak, D. H., R. S. Hedin, H. M. Edenborn, and P. E. McIntire. 1992. Treatment of metal-contaminated water using bacterial sulfate reduction: results from pilot-scale reactors. Biotechnology and Bioengineering 40:609–616.
Faulkner, S. P. and C. J. Richardson. 1990. Iron and managanese fractionation in constructed wetlands receiving acid mine drainage. p. 441–450.In P. F. Cooper and B. C. Findlater (eds.) Constructed Wetlands in Water Pollution Control. Pergamon Press, Oxford, U.K.
Gerber, D. W., J. E. Burris, and R. W. Stone. 1985. Removal of dissolved iron and manganese ions by aSphagnum moss. p. 365–372.In R. Brooks, D. Samuel, and J. Hill (eds.) Wetlands and Water Management on Mined Lands. The Pennsylvania State University, University Park, PA, USA.
Gotoh, S. and W. H. Patrick, Jr. 1972. Transformation of manganese in a waterlogged soil as affected by redox potential and pH. Soil Science Society of America Proceedings 36:738–742.
Hedin, R. S. and R. W. Nairn. 1993. Contaminant removal capabilities of wetlands constructed to treat coal mine drainage. p. 187–195.In G. A. Moshiri (ed.) Constructed Wetlands for Water Quality Improvement. Lewis Publishers, Boca Raton, FL, USA.
Kleinmann, R. L. P. and R. S. Hedin. 1993. Treat mine water using passive methods. Pollution Engineering, Aug:20–22.
Letterman, R. D. and W. J. Mitsch. 1978. Impact of mine drainage on a mountain stream in Pennsylvania. Environmental Pollution 17:53–73.
Lindsay, W. L. 1979. Chemical Equilibria in Soils. Wiley, New York, NY, USA.
Lovley, D. R. and E. J. P. Phillips. 1987. Competitive mechanisms for inhibition of sulfle reduction and methane production in the zone of ferric iron reduction in sediments. Applied Environmental Microbiology 53:2636–2641.
McIntire, P. and H. Edenborn. 1990. The use of bacterial sulfate reduction in the treatment of drainage from coal mines. pp. 409–415.In J. Skousen, J. Sencindiver & D. Samuel (eds.) Proceedings of the 1990 Mining and Reclamation Conference and Exhibition, Vol. II. West Virginia University, Morgantown, WV, USA.
Michaud, S. C. and C. J. Richardson. 1989. Relative radial oxygen loss in five wetland plants. p. 501–507.In D. A. Hammer (ed.) Constructed Wetlands for Wastewater Treatment. Lewis Publishers, Inc., Chelsea, MI, USA.
Miller, W. P., D. C. Martens, and L. W. Zelazny. 1986. Effects of sequence in extraction of trace metals from soils. Soil Science Society of America Journal 50:598–601.
Nirel, P. M. V., and F. M. M. Morel. 1990. Pitfalls of sequential extractions. Water Research 24:1055–1056.
Rauret, G., R. Rubio, J. F. López-Sánchez, and E. Casassas. 1988. Determination and speciation of copper and lead in sediments of a Mediterranean river (River Tenes, Catalonia, Spain). Water Research 22:449–455.
Rendel, P. S., G. E. Batley, and A. J. Cameron. 1980. Adsorption as a control of metal concentrations in sediment extracts. Environmental Science and Technology 14:314–318.
Robinson, G. E. 1984. Sequential chemical extractions and metal partitioning in hydrous Mn−Fe coatings: reagent choice and substrate composition affect results. Chemical Geology 47:97–112.
Rudd, T., J. A. Campbell, and J. N. Lester. 1988. The use of model compounds to elucidate metal forms in sewage sludge. Environmental Pollution 50:225–242.
Saeki, K., M. Okazaki, and S. Matsumoto. 1993. The chemical phase changes in heavy metals with drying and oxidation of the lake sediments. Water Research 27:1243–1251.
Sencindiver, J. C. and D. K. Bhumbla. 1988. Effects of Cattails (Typha) on metal removal from mine drainage. p. 359–366.In Mine Drainage and Surface Mine Reclamation. Vol. 1: Mine Water and Mine Waste. Bureau of Mines Information Circular 9183, Pittsburgh, PA, USA.
Shuman, L. M. 1985. Fractionation method for soil microelements. Soil Science 140:11–22.
Stark, L. R., W. R. Wenerick, P. J. Wuest, F. M. Williams, and S. E. Stevens, Jr. 1991. Adding a carbon supplement to simulated treatment wetlands improves water quality. p. 465–483.In L. Sirois (ed.) The Abatement of Acidic Drainage. Montreal, Quebec, Canada.
Stark, L. R., F. M. Williams, S. E. Stevens, Jr., and D. P. Eddy. 1994. Iron retention and vegetative cover at the Simco constructed wetland: an appraisal through year eight of operation. p. 89–98.In Proceedings, International Land Reclamation and Mine Drainage Conference and Third International Conference on the Abatement of Acidic Drainage, Pittsburgh, PA, USA.
Tarutis, W. J., Jr. 1989. Behavior of Iron and Managenese in the Sedimen: of a Wetland Subjected to Acidic Mine Drainage. M.S. Thesis, The Pennsylvania State University, University Park, PA, USA.
Tarutis, W. J., Jr. and R. F. Unz. 1990. Chemical diagenesis of iron and manganese in constructed wetlands receiving acidic mine drainage. p. 429–440.In P. F. Cooper and B. C. Findlater (eds.) Constructed Wetlands in Water Pollution Control. Pergamon Press, Oxford, U.K.
Tarutis, W. J., Jr. and R. F. Unz. 1994a. Soil color variations along an iron oxide gradient in a mine drainage treatment wetland. Wetlands 14:243–246.
Tarutis, W. J., Jr. and R. F. Unz. 1994b. Using decomposition kinetics to model the removal of mine water pollutants in constructed wetlands. Water Science and Technology 29:219–226.
Tarutis, W. J., Jr., R. F. Unz, and R. P. Brooks. 1992. Behavior of sedimentary Fe and Mn in a natural wetland receiving acidic mine drainage. Pennsylvania, U.S.A. Applied Geochemistry 7:77–85.
Taylor, H. N., K. D. Choate, and G. A. Brodie. 1993. Storm event effects on constructed wetlands discharges. p. 139–145.In G. A. Moshiri (ed.) Constructed Wetlands for Water Quality Improvement. Lewis Publishers, Boca Raton, FL, USA.
Tessier, A., P. G. C. Campbell, and M. Bisson. 1979. Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry 51:844–851.
Wieder, R. K. 1988. Determining the capacity for metal retention in man-made wetlands constructed for treatment of coal mine drainage. p. 375–381.In Mine Drainage and Surface Mine Reclamation. Vol. 1: Mine Water and Mine Waste. Bureau of Mines Information Circular 9183, Pittsburgh, PA, USA.
Wieder, R. K. 1989. A survey of constructed wetlands for acid coal mine drainage treatment in the eastern United States. Wetlands 9:299–315.
Wieder, R. K. 1992. The Kentucky Wetlands Project: a field study to evaluate man-made wetlands for acid coal mine drainage treatment. Final Report, U.S. Office of Surface Mining, Reclamation and Enforcement. Pittsburgh, PA, USA.
Wieder, R. K., M. Linton, and K. Heston. 1990. Laboratory mesocosm studies of Fe, Al, Mn, Ca, and Mg dynamics in wetlands exposed to synthetic acid coal mine drainage. Water, Air, and Soil Pollution 51:181–196.
Zadow, J. G. 1986. Utilisation of milk products: whey. p. 273–316.In: R. K. Robinson (ed.) Modern Dairy Technology, vol 1. Advances in Milk Processing. Elsevier Applied Science Publishers, New York, NY, USA.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stark, L.R., Williams, F.M., Wenerick, W.R. et al. The effects of carbon supplementation and plant species on iron retention in mesocosm treatment wetlands. Wetlands 15, 58–67 (1995). https://doi.org/10.1007/BF03160680
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03160680