Abstract
The purpose of this study was to examine the distribution of neuronal damage following transient cerebral ischemia in the rat model of four-vessel occlusion utilizing light microscopy as well as45Ca-autoradiography. Transient ischemia was induced for 30 min. The animals were allowed to survive for 7 d after ischemia.
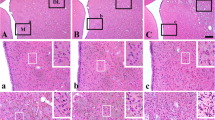
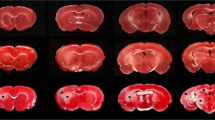
In the animals subjected to ischemia, the most frequently and seriously damaged areas were the paramedian region of hippocampus, the hippocampal CA1 sector, and the dorsolateral part of striatum, followed by the inferior colliculus, the substantia nigra, the frontal cortex, and the thalamus, which were moderate damaged. Furthermore, the cerebellar Purkinje neurons, the hippocampal CA4 sector, the medial geniculate body, and the hippocampal CA3 sector were slightly affected.45Ca-autoradiographyic study also revealed calcium accumulation in the identical sites of ischemic neuronal damage, except for the frontal cortex. Regional cerebral blood flow during 10 min of ischemia was severely decreased in selectively vulnerable areas. The blood flow in the medial geniculate body, the substantia nigra, the inferior colliculus, and the cerebellum was less pronounced than that in the selectively vulnerable areas. The present study demonstrates that transient cerebral ischemia can produce significant neuronal damage not only in the selectively vulnerable regions, but also in the brainstem.
Similar content being viewed by others
References
Araki T., Kato H., and Kogure K. (1989) Selective neuronal vulnerability following transient cerebral ischemia in the gerbil: distribution and time course.Acta Neurol. Scand. 80, 548–553.
Benveniste H. And Diemer N. H. (1988) Early postischemic45Ca accumulation in rat dentate hilus.J. Cereb. Blood Flow Metab. 8, 713–719.
Benveniste H., Drejer J., Schousboe A., and Diemer N. H. (1984) Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis.J. Neurochem. 43, 1369–1374.
Brierley J. (1976) Cerebral hypoxia. Greenfield’s Neuropathology, (Blackwood W. and Corsellis A., eds.), pp. 43–85, Edward Arnold, London.
Cotman C. W., Monaghan D. T., Ottersen O. P., and Storm-Mathisen J. (1987) Anatomical organizational of excitatory amino acid receptors and their pathways.Trend. Neurosci. 10, 273–280.
Crain B. J., Westerkam W. D., Harrison A. H., and Nadler J. V. (1988) Selective neuronal death after transient forebrain ischemia in the mongolian gerbil: A silver impregnation study.Neuroscience 27, 387–402.
Diemer N. H. and Siemkowicz E. (1980) Increased 2-deoxyglucose uptake in hippocampus, globus pallidus and substantia nigra after cerebral ischemia.Acta Neurol. Scand. 61, 56–63.
Dienel G. A. (1984) Regional accumulation of calcium in postischemic rat brain.J. Neurochem. 43, 913–925.
Jorgensen M. B. and Diemer N. H. (1982) Selective neuron loss after cerebral ischemia in the rat: Possible role of transmitter glutamate.Acta Neurol. Scand. 66, 536–546.
Kogure K., Tanaka J., and Araki T. (1988) The mechanism of ischemia-induced brain cell injury.Neurochem. Pathol. 9, 145–170.
Onodera H., Araki T., and Kogure K. (1989) Protein kinase C activity in the rat hippocampus after forebrain ischemia: autoradiographic analysis by [3H]phorbol 12, 13-dibutyrate.Brain Res. 481, 1–7.
Paxinos G. and Watson C. (1982)The Rat Brain in Stereotaxic Coordinates. Academic, Sydney.
Pulsinelli W. A. (1985) Selective neuronal vulnerability: morphological and molecular characteristics.Progress in Brain Research, vol. 63 (Kogure K., Hossmann K.-A., Siesjö B. K., and Welsh F. A., eds.), pp. 29–37, Elsevier, New York.
Pulsinelli W. A. and Brierley J. B. (1979) A new model of bilateral hemispheric ischemia in the unanesthetized rat.Stroke 10, 267–272.
Pulsinelli W. A., Brierley J. B., and Plum F. (1982) Temporal profile of neuronal damage in a model of transient forebrain ischemia.Ann. Neurol. 11, 491–498.
Pulsinelli W. A., Brierley J. B., Duffy T., Levy D., and Plum F. (1981) Ischemic neuronal damage, postischemic regional blood flow, and glucose metabolism in rat brain.J. Cereb. Blood Flow Metab. 1, 166–167.
Rothmann S. M. and Olney J. W. (1986) Glutamate and the pathophysiology of hypoxic-ischemic brain damage.Ann. Neurol. 19, 105–111.
Sakamoto N., Kogure K., Kato H., and Ohtomo H. (1985) Disturbed Ca2+ homeostatis in the gerbil hippocampus following brief transient ischemia.Brain Res. 364, 372–376.
Sakurada O., Kennedy C., Jehle J. W., Brown J. D., Carbin L., and Sokoloff L. (1978) Measurement of local cerebral blood flow with iodo-14C-antipyrine.Am. J. Physiol. 234, H59-H66.
Siman R., Noszek J. C., and Kegerise C. (1989) Calpain I activation is specifically related to excitatory amino acid induction of hippocampus damage.J. Neurosci. 9, 1579–1590.
Smith M.-L., Auer R. N., and Siesjö B. K. (1984) The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia.Acta Neuropathol. (Berlin) 64, 319–332.
Wieloch T. (1985) Neurochemical correlates to selective neuronal vulnerability.Progress in Brain Research, (Kogure K., Hossmann K.-A., Siesjö B. K., and Welsh F. A., eds.), vol 63, pp. 69–85, Elsevier, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Araki, T., Inoue, T., Kato, H. et al. Neuronal damage and calcium accumulation following transient cerebral ischemia in the rat. Molecular and Chemical Neuropathology 12, 203–213 (1990). https://doi.org/10.1007/BF03159945
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03159945