Abstract
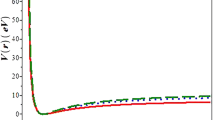
The dissociation energies of the diatomic molecules GaF, InF and TlF have been computed by fitting empirical potential functions to the true potential energy curves for the electronic ground states of the molecules. The five parameter Hulburt-Hirschfelder function and Rao and Reddy's modified potential function have been used. The estimated dissociation energies are 9.772±0.288, 8.571±0.256 and 7.209±0.641 kJ/mol for GaF, InF and TlF, respectively. These values are in good agreement with literature values. The first ionization potentials for these molecules are estimated.
Similar content being viewed by others
References
G. R. Somayajulu, J. Chem. Phys.,33, 1541, 1960.
S. N. Thakur, R. B. Singh and D. K. Rai, J. Sci. and Ind. Res.,27, 339, 1968.
R. F. Barrow, Trans. Farad. Soc.,56, 952, 1960.
J. Singh, K. P. R. Nair and D. K. Rai, J. Mol. Struct.,6, 328, 1970.
D. Steele, E. R. Lippincott and J. T. Vanderslice, Rev. Mod. Phys.,34, 239, 1962.
T. V. Ramakrishna Rao and R. Ramakrishna Reddy, JQSRT,25, 295, 1981.
Mizushima Masataka, Rotating Diatomic Molecules, John Wiley, New York, 1975.
H. M. Hulburt and J. O. Hirschfelder, J. Chem. Phys.,9, 61, 1941.
R. R. Reddy and T. V. R. Rao, J. Mol. Struct., (Theor. Chem.),90, 49, 1982.
R. R. Reddy and T. V. R. Rao, Acta Phys. Pol.64A, 309, 1983.
T. V. R. Rao, R. R. Reddy and A. Subbarami Reddy, J. Mol. Struct., (Theor. Chem.),105, 249, 1983.
S. V. J. Lakshman and T. V. Ramakrishna Rao, J. Phys., B4, 269, 1971.
A. G. Gaydon, Dissociation Energies, Chapman and Hall, London, 1968.
K. P. Huber and G. Herzberg, Molecular Spectra and Molecular Structure, Vol. IV, Constants of Diatomic Molecules, Van Nostrand Reinhold, New York, 1979.
E. Murad, D. L. Hildenbrand and R. P. Main, J. Chem. Phys.,45, 263, 1966.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reddy, R.R., Rao, T.V.R. Dissociation energies for the electronic ground states of GaF, InF and TlF. Acta Physica Hungarica 58, 169–173 (1985). https://doi.org/10.1007/BF03155710
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03155710