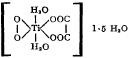Summary
-
1.
The method of the preparation of the normal peroxy titanium oxalate TiO2C2O4, 3·5 H2O is described. If an excess of hydrogen peroxide is not maintained in the starting solution, substances with the molar compositions Ti2O3 (C2O4)2, 7 H2O and Ti1·5O2·5 (C2O4)1·5, 5·5 H2O are obtained.
-
2.
It has been shown by the various physico-chemical studies that the substance TiO2C2O4, 3·5 H2O is a true peroxy complex, while the other two substances are mixtures of the peroxy complex and its deoxygenated product (basic oxalate TiOC2O4) in different proportions.
-
3.
The keeping quality of the peroxy complex indicates that the complex loses the peroxy oxygen gradually, transforming itself into the basic oxalate.
-
4.
Molecular weights of the three complexes described in (1) have been determined cryoscopically.
-
5.
Conductivity of the aqueous solution of the normal peroxy complex gave the average dissociation constant K = 4·5 × 10-2.
-
6.
Spectrophotometric investigations on the peroxy complex showed that the peroxy complex has the atomic ratio of Ti : O (peroxy) as 1:1 and the absorption maximum of the complex is at the wavelength 425 mµ.
-
7.
Potentiometric titration of the aqueous solution of the complex against sodium hydroxide indicated the dibasic character of the complex in the solution.
-
8.
Vapour pressure data for the normal peroxy complex gave the heat of dissociation equal to 15·4 K.cal./mole of water, indicating that the water associated with the complex is combined one.
-
9.
Oxygen pressure for the normal peroxy complex varied from 0·12 to 0·30 mm. of mercury in the temperature range 2° to 45° C.
-
10.
Dehydration studies on the normal peroxy titanium oxalate complex in vacuum showed that out of 3·5 moles of water, 1·5 moles are removed from the complex while 2 moles are retained with the complex.
-
11.
Decomposition products obtained on heating the peroxy complex in vacuum are carbon dioxide, carbon monoxide, oxygen, water, titanium dioxide and carbon.
-
12.
The complex has been assigned the structure
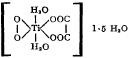
based on various physico-chemical studies.
Similar content being viewed by others
References
Mazzucchelli, A. and PotanelliAtti. Acad. Lincei, 1908,18(1), 518.
Kharkar, D. P. and Patel, C. C.Curr. Sci., 1955,24, 413.
Weissler, A.Ind. Eng. Chem. (Anal. Ed.), 1945,17, 695.
Kolthoff, I. M. and Sandell, E. B.Text-book of Quantitative Inorganic Analysis, Macmillan Co., New York, 1943, p. 630.
Abel, E.Z. Physikal. Chem., 1931,154 A, 167.
Emeléus, H. J. and Anderson, J. S.Modern Aspects of Inorganic Chemistry, Routledge and Kegan Paul Ltd., London, 1947, pp. 351.
Krauss, F. and Oettner, C.Z. anorg. allg. Chem., 1934,218, 21.
Liebhafsky, H. A. —, 1934,221, 25.
Murthy, A. R. V., Bharadwaj, D. S. and Mallya, R. M.Chemistry and Industry, April 28, 1956, pp. 300.
Ephraim, F.Inorganic Chemistry, Gurney and Jackson, London, 1949, pp. 295.
Welo, L. A. and Baudisch, O.Nature, 1925,116, 606.
Guoy, M.Compt. rend., 1889,109, 935.
Pauling, L.J.A.C.S., 1931,53, 1367.
Author information
Authors and Affiliations
Additional information
Communicated by Dr. M. R. A. Rao,f.a.sc.
Rights and permissions
About this article
Cite this article
Kharkar, D.P., Patel, C.C. Peroxy titanium oxalate. Proc. Indian Acad. Sci. 44, 287–306 (1956). https://doi.org/10.1007/BF03046055
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03046055