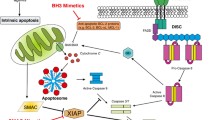
Abstract
Death ligands (TNF, FasL, TRAIL) and their respective death receptor signaling pathways can be used to induce tumor cells to undergo apoptosis. Chemotherapeutic drugs can induce apoptosis and the upregulation of death ligands or their receptors. Downstream events following cytotoxic stressinduced DNA damage and the signaling pathways that lead to the induction of apoptosis may be either dependent or independent of death receptor signaling. The involvement of the Fas signaling pathway in chemotherapy-induced apoptosis has been the most extensively studied, with the current emergence of information on the TRAIL signaling pathway. Fas-mediated and chemotherapy-induced apoptosis can converge at the level of the receptor, FasL, DISC formation, activation of the initiator caspase-8, at the level of the mitochondria, or at the level of downstream effector caspase activation. Convergence is influenced by the specific form of DNA damage, the cellular environment, and the specific pathway(s) by which death receptor-mediated or drug-mediated apoptosis are induced. This review discusses the different levels of interaction between signaling pathways in the different forms of cell death.
Similar content being viewed by others
References
Ashkenazi A, Dixit VM: Death receptors: Signaling and modulation. Science 281:305–108, 1998.
Ware CF, VanArsdale S, VanArsdale TL: Apoptosis mediated by the TNF-related cytokine and receptor families. J Cell Biochem 60:47–55, 1996.
McGeehan GM, Becherer JD, Bast RCJr, et al: Regulation of tumor necrosis factor-alpha processing by a metalloproteinase inhibitor. Nature 370:558–561, 1994.
Tanaka M, Itai T, Adachi M, et al: Downregulation of Fas ligand by shedding. Nature Med 4:31–36, 1998.
Kayagaki N, Kawasaki A, Ebata T, et al: Metalloproteinasemediated release of human Fas ligand. J Exp Med 182:1777–1783, 1995.
Ashkenazi A, Dixit VM: Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 11:255–260, 1999.
Nagata S, Golstein P:The Fas death factor. Science 276:1449, 1995.
Aral H, Gordon D, Nabel EG, et al: Gene transfer of Fas ligand induces tumor regression in vivo. Proc Natl Acad Sci USA 94:13862–13867, 1997.
French LE, Hahne M, Viard I, et al: Fas and Fas ligand in embryos and adult mice: ligand expression in several immuneprivileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol 133:335–343, 1996.
Moller P, Koretz K, Leithauser F, et al: Expression of Apo-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer 57:371–377, 1994.
Pitti RM, Marsters SA, Lawrence DA, et al: Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 396:699–703. 1998.
Itoh N, Yonehara S, Ishii A Itoh N, Yonehara S, Ishii A, et al: The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 66:233–243, 1991.
Boldin MP, Varfolomeev EE, Pancer Z, et al: A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem 270:7795–7798, 1995.
Chinnaiyan AM, O’Rourke K, Tewari M, et al: FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81:505–512, 1995.
Boldin MP, Goncharov TM, Goltsev YV, et al: Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO1 and TNF receptor-induced cell death. Cell 85:803–815, 1996.
Medema JP, Scaffidi C, Kischkel FC, et al: FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J 16:2794–2804, 1997.
Muzio M, Chinnaiyan AM, Kischkel FC, et al: FLICE a novel FADD-homologous ICE/CED-3-like protease is recruited to the CD95 (Fas/Apo-1) death-inducing signaling complex. Cell 85:817–827, 1996.
Irmler M, Thome M, Hahne M, et al: Inhibition of death receptor signals by cellular FLIP. Nature 388:190–195. 1997.
Scaffidi C, Schmitz I, Krammer PH, et al: The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem 274:1541–1548. 1999.
Scaffidi C, Fulda S, Srinivasan A, et al: Two CD95 (APO1/Fas) signaling pathways. EMBO J 17:1675–1687, 1998.
Sun X-M, MacFarlane M, Zhuang J, et al: Distinct caspase cascades are initiated in receptor-mediated and chemicalinduced apoptosis. J Biol Chem 274:5053–5060, 1999.
Kuwana T, Smith JJ, Muzio M, et al: Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J Biol Chem. 27:16589–16594, 1998.
Li P, Nijhawan D, Alnemri ES, Wang X: Cytochrome c and dATP-dependent formation of Apaf-l/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489, 1997.
Jeremias I, Herr I, Boehler T, et al: TRAIL/Apo-2-ligandinduced apoptosis in human T cells. Eur J Immunol 28:143–152, 1998.
Marsters SA, Pitti RM, Donahue CJ, et al: Activation of apoptosis by Apo-2 ligand is independent of FADD but blocked by CrmA. Curr Biol 6:750–752, 1996.
Griffith TS, Lynch DH: TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol 10:559–563, 1998.
Zhang XD, Franco AV, Nguyen T, et al: Differential Localization and Regulation of Death and Decoy Receptors for TNF-Related Apoptosis-Inducing Ligand (TRAIL) in Human Melanoma Cells. J Immunol 164:3961–3970, 2000.
Walczak H, Miller RE, Ariail K, et al: Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nature Med 5:157–163, 1999.
Walczak H, Degli-Eposti MA, Johnson RS, et al: TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J 16:5386–5397, 1997.
Wiley SR, Schooley K, Smolak PJ, et al: Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3:673–682, 1995.
Pan G, O’Rourke K, Chinnaiyan AM, et al: The receptor for the cytotoxic ligand TRAIL. Science 276:111–113, 1997.
Pan G, Ni J, Wei Y-F, et al: An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277:815–818, 1997.
Sheridan JP, Marsters SA, Pitti PM, et al: Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277:818–821, 1997.
Degli-Eposti MA, Smolak PJ, Walczak H, et al: Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med 186:1165–1170, 1997.
Degli-Eposti MA, Dougall WC, Smolak PJ, et al: The novel receptor TRAIL-R4 induces NF-kB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 7:813–820, 1997.
Marsters SA, Sheridan JP, Pitti RM, et al: A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol 7:1003–1006, 1997.
Frank S, Kohler U, Schackert G, et al: Expression of TRAIL and its receptors in human brain tumors. Biochem Biophys Res Commun 257:454–459, 1999.
Griffith TS, Chin WA, Jackson GC, et al: Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol 161:2833–2840, 1998.
Thomas WD, Hersey P: TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J Immunol 161:2195–2200, 1998.
Ashkenazi A, Pai RC, Fong S, et al: Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 104:155–162, 1999.
Rieger J, Naumann U, Glaser T, et al: APO2 ligand: a novel weapon against malignant glioma? FEBS Lett 427:124–128, 1998.
Schneider P, Thome M, Burns K, et al: TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity 7:831–6, 1997.
Schneider P, Thome T, Burns K, et al: TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity 7:831–836, 1997.
Kischkel FC, Lawrence DA, Chuntharapai A, et al: Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 12:611–620, 2000.
Sprick MR, Weigand MA, Rieser E, et al: FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12:599–609, 2000.
Bodmer JL, Holler N, Reynard S, et al: TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol 2:241–243, 2000.
Hickman JA: Apoptosis induced by anticancer drugs. Cancer Metast Rev 11:121–139, 1992.
Holzman D: Apoptosis provides new targets for chemotherapy. J Natl Cancer Inst 88:1098–1100, 1996.
Sellers WR, Fisher DE: Apoptosis and cancer drug targeting. J Clin Invest 104:1655–1661, 1999.
Miyashita T, Reed JC: Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood 81:151–157, 1993.
Bunz F, Hwang PM, Torrance C, et al: Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest 104:263–269, 1999.
Zhu H, Fearnhead HO, Cohen GM: An ICE-like protease is a common mediator of apoptosis induced by diverse stimuli in human monocytic THP.l cells. FEBS LETT 375:303–308, 1995.
Petak I, Mihalik R, Bauer PI, et al: BCNU is a caspase-mediated inhibitor of drug-induced apoptosis. Cancer Res 58:614–618, 1998.
Petak I, Tillman, DM, Harwood FG, et al: Fas-dependent and -independent mechanisms of cell death following DNA damage in human colon carcinoma cells. Cancer Res 60:2643–2650. 2000.
Tounekti O, Pron G, Belehradek J, et al: Bleomycin, an apoptosis-mimetic drug that induces two types of cell death depending on the number of molecules internalized. Cancer Res 53:5462–5469, 1993.
Skladanowski A, Konopa, J: Adriamycin and daunomycin induce programmed cell death (apoptosis) in tumour cells. Biochem Pharmacol 46:375–382, 1993.
Pellicciari C, Bottone MG, Schaack V, et al: Etoposide at different concentrations may open different apoptotic pathways in thymocytes. Eur J Histochem 40:289–298, 1996.
Lock RB, Stribinskiene L: Dual modes of death induced by etoposide in human epithelial tumor cells allow Bcl-2 to inhibit apoptosis without affecting clonogenic survival. Cancer Res 56:4006–4012, 1996.
Kasibhatla S, Brunner T, Genestier L, et al: DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell 1, 543–551. 1998.
Faris M, Kokot N, Latinis K, et al: The c-Jun N-terminal kinase cascade plays a role in stress-induced apoptosis in Jurkat cells by up-regulating Fas ligand expression. J Immunol 160:134–144, 1998.
Friesen C, Herr I, Krammer PH, et al: Involvement of the CD95 (APO-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nature Med 2:574–577, 1996.
Fulda S, Sievens H, Frieson C, et al: The CD95 (APO l-Fas) system mediates drug-induced apoptosis in neuroblastoma cells. Cancer Res 57:3823–3829, 1997.
Villunger A, Egle A, Kos M, et al: Drug-induced apoptosis is associated with enhanced Fas (APO-1/CD9J) ligand expression but occurs independently of Fas (APO-1/CD95) signaling in human T-acute lymphatic leukemia cells. Cancer Res 57:3331–3334, 1997
Eischen CM, Kottke TJ, Martins LM, et al: Comparison of Apoptosis in wild-type and Fas-resistant cells: chemotherapyinduced apoptosis is not demendent on Fas/Fas ligand interactions. Blood 90:935–943, 1997.
Wen J, Ramadevi N, Nguyen D, et al: Antileukemic drugs increase death receptor 5 levels and enhance apo-2L-induced apoptosis of human acute leukemia cells. Blood 96:3900–3906, 2000.
Nagane M, Pan G, Weddle JJ, et al: Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factorrelated apoptosis-inducing ligand in vitro and in vivo. Cancer Res 60:847–853, 2000.
Keane MM, Ettenberg SA, Nau MM, et al: Chemotherapy augmants TRAIL-induced apoptosis in breast cell lines. Cancer Res 59:734–741, 1999.
Alderson MR, Tough TW Davissmith T, et al: Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med 181:71–77, 1995.
Brunner T, Mogil RJ, La Face D, et al: Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 373:441–444, 1995.
Fulda S, Strauss G, Meyer E, et al: Functional CD95 ligand and CD95 death-inducing signaling complex in activation-induced cell death and doxorubicin-induced apoptosis in leukemic T cells. Blood 95:301–308, 2000.
Fulda S, Meyer E, Friesen C, et al: Cell type specific involvement of death receptor and mitochondrial pathways in druginduced apoptosis. Oncogene 20:1063–1075, 2001.
Houghton JA, Harwood FG, Tillman DM: Thymineless death in colon carcinoma cells is mediated via Fas signaling. Proc Natl Acad Sci USA 94:8144–8149, 1997.
Tillman DM, Petak I, Houghton JA: A Fas-dependent component in 5-fluorouracil/leucovorin-induced cytotoxicity in colon carcinoma cells. Clin Cancer Res 5:425–430. 1999.
Eichhorst ST, Muller M, Li-Weber M, et al: A novel AP-1 element in the CD95 ligand promoter is required for induction of apoptosis in hepatocellular carcinoma cells upon treatment with anticancer drugs. Mol Cell Biol 20:7826–7837, 2000.
Eichhorst ST, Muerkoster S, Weigand MA, et al: The chemotherapeutic drug 5-fluorouracil induces apoptosis in mouse thymocytes in vivo via activation of the CD95(APO-1/Fas) system. Cancer Res 61:243–248. 2001.
Harwood FG, Kasibhatla S, Petak I, et al: Regulation of FasL by NF-kappaB and AP-1 in Fas-dependent thymineless death of human colon carcinoma cells. J Biol Chem 275:10023–10029. 2000.
Muller M, Wilder S, Bannasch D, et al: P53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer agents. J Exp Med., 188:2033–2045, 1998.
Petak I, Tillman DM, Houghton JA: P53-dependence of Fas induction amd acute apoptosis in response to 5-fluorouracilleucovorin in human colon carcinoma cell lines. Clin Cancer Res 6:4432–4441, 2000.
Siltonen T, Mantymaa P, Saily M, et al: Etoposide-induced apoptosis is not associated with the fas pathway in acute myeloblastic leukemia cells. Leukemia Res 24:281–288, 2000.
Tolomeo M, Dusonchet T, Meli M, et al: The CD95/CD95 ligand system is not the major effector in anticancer drug-mediated apoptosis. Cell Death Diff 5:735–742, 1998.
Shao R-G, Cao C-X, Nieves-Neira W, et al: Activation of the Fas pathway independently of Fas ligand during apoptosis induced by camptothecin in p53 mutant colon carcinoma cells. Oncogene 20:1852–1859, 2001.
Wieder T, Essmann F, Prokop A, et al: Activation of caspase-8 in drug-induced apoptosis of B-lymphoid cells is independent of CD95/Fas receptor-ligand interaction and occurs downstream of caspase-3. Blood 97:1378–1387, 2001.
Wesselborg S, Engels IH, Rossmann E, et al: Anticancer drugs induce caspase-8/FLICE activation and apoptosis in the absence of CD95 receptor/ligand interaction. Blood 93:3053–3063, 1999.
Tang D, Lahti JM, Kidd VJ: Caspase-8 activation and Bid cleavage contribute to MCF7 cellular execution in a caspase3-dependent manner during staurosporine-mediated apoptosis. J Biol Chem 275:9303–9307, 2000.
Ferreira CG, Span SW Peters GJ: Chemotherapy triggers apoptosis in a caspase-8-dependent and mitochondria-controlled manner in the non-small cell lung cancer cell line NCIH460. Cancer Res 60:7133–7141, 2000.
Kinoshita H, Yoshikawa H, Shiiki K, et al: Cisplatin (CDDP) sensitizes human osteosarcoma cells to Fas/CD95-mediated apoptosis by downregulating FLIP-L expression. In J Cancer 88:986–991, 2000.
Tang D, Lahti JM, Grenet J, et al: Cycloheximide-induced T-cell death is mediated by a Fas-associated death domaindependent mechanism. J Biol Chem 274:7245–7252, 1999.
Dorrie J, Schuh W Keil A, et al: Regulation of CD95 expression and CD95-mediated cell death by interferon-gamma in acute lymphoblastic leukemia with chromosomal translocation t(4;ll). Leukemia 13:1539–1547, 1999.
Griffith TS, Chin WA, Jackson GC, et al: Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol 161:2833–2840, 1998.
Bretz JD, Rymaszewski M, Arscott PL, et al: TRAIL death pathway expression and induction in thyroid follicular cells. J Biol Chem 274:23627–23632, 1999.
Kataoka T, Schroter M, Hahne M, et al: FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma radiation. J Immunol 161:3936–3942, 1998.
Los M, Herr I, Friesen C, et al: Cross-resistance of CD95- and drug-induced apoptosis as a consequence of deficient activation of caspases (ICE/Ced-3 proteases). Blood 90:3118–3129, 1997.
Boesen-de Cock JG, Tepper AD, de Vries E, et al: Common regulation of apoptosis signaling induced by CD95 and the DNA-damaging stimuli etoposide and -radiation downstream from caspase-8 activation. J Biol Chem 274:14255–14261, 1999.
Sun X-M, MacFarlane M, Zhuang J, et al: Distinct caspase cascades are initiated in receptor-mediated and chemicalinduced apoptosis. J Biol Chem 274:50530–50560, 1999.
Wu X-Y, Mizutami Y, Kakehi Y, et al: Enhancement of Fasmediated apoptosis in renal cell carcinoma cells by adriamycin. Cancer Res 60:2912–2918, 2000.
Tamura T, Aoyama N, Saya H, et al: Induction of Fas-mediated apoptosis in p53-transfected human colon carcinoma cells. Oncogene, 11:1939–1946, 1995.
Owen-Sachaub LB, Zhang W Cusack JC, et al: Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol 15:3032–3040, 1995.
Rakkar ANS, Katayos Y, Kim M, et al: A novel adenoviral vector expressing human Fas/CD95/APO-l enhances p53-mediated apoptosis. Cell Death Diff 6:326–333, 1999.
Fukazawa T, Fujiwara T, Morimoto Y, et al: Differential involvement of the CD95 (Fas/APO-1) receptor/ligand system on apoptosis induced by the wild-type p53 gene transfer in human cancer cells. Oncogene, 18:2189–2199, 1999.
Shimizu M, Yoshimoto T, Nagata S, et al: A trial to kill tumor cells through Fas (CD95)-mediated apoptosis in vivo. Biochem Biophys Res Commun 228:375–379, 1996.
Wu GS, Burns TF, McDonald ER 3rd,et al: KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet 17:141–143, 1997.
Nagane M, Pan G, Weddle JJ, et al: Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factorrelated apoptosis-inducing ligand in vitro and in vivo. Cancer Res 60:847–853, 2000.
Herr I, Wilhelm D, Bohler T, et al: JNK/SAPK activity is not sufficient for anticancer therapy-induced apoptosis involving CD95-L, TRAIL and TNF-alpha. Int J Cancer 80:417–424, 1999.
Friesen C, Fulda S, Debatin KM: Induction of CD95 ligand and apoptosis by doxorubicin is modulated by the redox state in chemosensitiveand drug-resistant tumor cells. Cell Death Differ 6:471–480, 1999.
Herr I, Wilhelm D, Bohler T, et al: Activation of CD95 (APO1/Fas) signaling by ceramide mediates cancer therapy-induced apoptosis. EMBO J 16:6200–6208, 1997.
Boland MP, Foster SJ, O’Neill LAJ: Daunorubicin activates NF-kB and induces KB-dependent gene expression in HL-60 promyelocytic and Jurkat T lymphoma cells. J Biol Chem 272:12952–12960, 1997
Miyamoto S, Verma IM: Rel/NF-kappaB/IkappaB story. Adv Cancer Res 66:255–292, 1995.
Baldwin AS: The NF-kappaB and I kappaB proteins: new discoveries and insights. Ann Rev Immunol 14:649–683, 1996.
Chen Z, Hagler J, Palombella VJ, et al: Signal-induced sitespecific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes and Dev 9:1586–1597, 1995.
Karin M:The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 270:16483–16486, 1995.
Kyriakis J, Banerjee P, Nikolakaki E, et al: The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369:156–160, 1994.
Minden A, Lin A, McMahon M, et al: Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science 266:1719–1723, 1994.
Derijard B, Hibi M, Wu I-H, et al: JNK 1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025–1037, 1994.
Chen Y-R, Wang X, Templeton D, et al: The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and radiation. J Biol Chem 271:31929–31936, 1996.
Kharbanda S, Ren R, Pandey P, et al: Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature 376:785–788, 1995.
Van Dam H, Wilhelm D, Herri, I, et al: ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J 14:1798–1811, 1995.
Yu R, Shtil AA, Tan T-H, et al: Adriamycin activates c-jun Nterminal kinase in human leukemia cells: a relevance to apoptosis. Cancer Lett 107:73–81, 1996.
Scimiya H Mashima T, Toho M, et al: c-Jun NH2-terminal kinase-mediated activation of interleukin-1β converting enzyme/CED-3-like protease during anticancer drug-induced apoptosis. J Biol Chem 272:4631–4636, 1997.
Sanchez-Perez I, Murguia JR, Perona R: Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene 16:533–540, 1998.
Rudel T, Zenke FT, Chuang T-H, et al: Cutting edge: p21-activated kinase (PAK) is required for Fas-induced JNK activation in Jurkat cells. J Immunol 160:7–11, 1998.
Liu Z-G, Baskaran C, Lee-Choe ET, et al: Three distinct signaling responses by murine fibroblasts to genotoxic stress. Nature 384:273–276, 1996.
Enari M, Sakahiera H, Yokoyama H, et al: A caspase-activated Dnase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43, 1998.
Lee FS, Hagler J, Chen CJ, et al: Activation of the IkBk kinase complex by MEKK1, a kinase of the JNK pathway. Cell 88:213–222, 1997.
Bessho R, Matsubara K, Kubota M, et al: Pyrrolidine dithiocarbamate, a potent inhibitor of nuclear factor kB (NF-kB) activation, prevents apoptosis in human promyelocytic leukemia HL-60 cells and thymocytes. Biochem Pharmacol 48:1883–1889, 1994.
Piret B, Piette J: Topoisomerase poisons activate the transcription factor NF-kB in ACH-2 and CEM cells, ucl Acids Res 24:4242–4248, 1996.
Brach MA, Kharbanda SM, Herrmann F, et al: Activation of the transcription factor kappaB in human KG-1 myeloid leukemia cells treated with 1-beta-D-arabinofuranosylcytosine. Mol Pharmacol 41:60–63, 1992.
Testi R. Sphingomyelin breakdown and cell fate. Trends Biochem Sci 21:468–471, 1996.
Bose R, Verheij M, Haimovitz-Friedman A, et al: Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell 82:405–414. 1995.
Hannun YA: Functions of ceramide in coordinating cellular responses to stress. Science 274:1855–1859, 1996.
Hannun YA, Obeid LM: Mechanisms of ceramide-mediated apoptosis. Adv Exp Med Biol 407:145–149, 1997.
Mathias S, Pena LA, Kolesnick RN: Signal transduction of stress via ceramide. Biochem J 335:465–480, 1998.
Verheij M, Bose R, Lin XH, et al: Requirement for ceramideinitiated SAPK/JNK signalling in stress-induced apoptosis. Nature 380:75–79, 1996.
Wiegmann K, Schutze S, Machleidt T, et al: Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell 78:1005–1015, 1994.
Spiegel S, Foster D, Kolesnick R: Signal transduction through lipid second messengers. Curr Opin Cell Biol 8:159–167, 1996.
Hirschberg K, Rodger J, Futerman AH: The long-chain sphingoid base of sphingolipids is acylated at the cytosolic surface of the endoplasmic reticulum in rat liver. Biochem J 290:751–757, 1993.
Shimeno H, Soeda S, Yasukouchi M, et al: Fatty acyl-Co A: sphingosine acyltransferase in bovine brain mitochondria: its solubilization and reconstitution onto the membrane lipid liposomes. Biol Pharm Bull 18:1335–1339, 1995.
Adam-Klages S, Schwandner R, Adam D, et al: Distinct adapter proteins mediate acid versus neutral sphingomyelinase activation through the p55 receptor for tumor necrosis factor. J Leukoc Biol 63:678–682, 1998.
Schwandner R, Wiegmann K, Bernardo K, et al: TNF receptor death domain-associated proteins TRADD and FADD signal activation of acid sphingomyelinase. J Biol Chem 273:5916–5922, 1998.
Wiegmann K, Schwandne R, Krut O, et al: Requirement of FADD for tumor necrosis factor-induced activation of acid sphingomyelinase. J Biol Chem 274:5267–5270, 1999.
Cuvillier O, Edsall L, Spiegel S: Involvement of sphingosine in mitochondria-dependent Fas-induced apoptosis of type II Jurkat T cells. J Biol Chem 275:15691–15700, 2000.
Cecconi F, Alvarez-Bolado G, Meyer BI, et al: Apafl (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 94:727–737, 1998.
Susin SA, Zamzami N, Castedo M, et al: The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-l/CD95and ceramide-induced apoptosis. J Exp Med 186:25–37, 1997.
De Maria R, Lenti L, Malisan F, et al: Requirement for GD3 ganglioside in CD95and ceramide-induced apoptosis. Science 277:1652–1655, 1997.
Zhou H, Summers SA, Birnbaum MJ, et al: Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem 273:16568–16575, 1998.
Boland MP, Foster SJ, O’Neill LA: Daunorubicin activates NFkappaB and induces kappaB-dependent gene expression in HL-60 promyelocytic and Jurkat T lymphoma cells. J Biol Chem 272:12952–12960, 1997.
Dbaibo GS, Pushkareva MY, Rachid RA, et al: p53-dependent ceramide response to genotoxic stress. J Clin Invest 102:329–339, 1998.
Sawai H, Okazaki T, Yamamoto H, et al: Requirement of AP-1 for ceramide-induced apoptosis in human leukemia HL-60 cells. J Biol Chem 270:27326–27331, 1995.
Scaffidi C, Schmitz I, Zha J, et al: Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem 274:22532–22538, 1999.
Cuvillier O, Pirianov G, Kleuser B, et al: Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381:800–803, 1996.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by NIH Awards RO1 CA 32613, the Cancer Center Support (CORE) Grant CA 21765, and by the American Lebanese Syrian Associated Charities. I. Petak was supported in part by a scholarship from the Fulbright Program, and by the 1st Institute of Pathology and Experimental Cancer Research, Semmelweis University, Budapest, Hungary
Rights and permissions
About this article
Cite this article
Petak, I., Houghton, J.A. Shared pathways: Death receptors and cytotoxic drugs in cancer therapy. Pathol. Oncol. Res. 7, 95–106 (2001). https://doi.org/10.1007/BF03032574
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03032574