Abstract
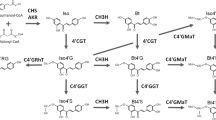
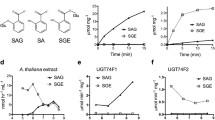
Biochemical characterization of the recombinant gene products from theArabidopsis glucosyltransferase multigene family has identified one enzyme with high activity toward the plant cellular regulator jasmonic acid (JA). The protein, AtJGT1 (UDP-glucose:JA glucosyltransferase), also has significant activities with other substrates, such as dihydrojasmonicacid, indole-3-acetic acid (IAA), indole-3-propionic acid, and indole-3-butyric acid. TheK M values of AtJGT1 for JA or IAA are similar to those of anArabidopsis IAA glucosyltransferase UGT84B1 previously reported. Northern blot analysis showed thatAtJGTI is highly expressed in the leaves, but only slightly detectable in the roots, stems, and inflorescences. This study describes the first biochemical analysis of a recombinant glucosyltransferase with JA activity, and provides the foundation for future genetic approaches to understanding the role of JA-glucose inArabidopsis.
Similar content being viewed by others
Literature Cited
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem72: 248–254
Cohen JD, Bandurski RS (1982) Chemistry and physiology of the bound auxins. Annu Rev Plant Physiol33: 403–430
Epstein E, Ludwig-Müller J (1993) lndole-3-butyric acid in plants: Occurrence, synthesis, metabolism, and transport. Physiol Plant88: 382–389
Guan KL, Dixon JE (1991) Eukaryotic proteins expressed inEscherichia coli: An improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem192: 262–267
Jackson RG, Kowalczyk M, Li Y, Higgins G, Ross J, Sandberg G, Bowles DJ (2002) Over expression of anArabidopsis gene encoding a glucosyltransferase of indole-3-acetic acid: Phenotypic characterization of transgenic lines. Plant J32: 573–583
Jackson RG, Lim EK, Li Y, Kowalczyk M, Sandberg G, Hoggett J, Ashford DA, Bowles DJ (2001) Identification and biochemical characterization of anArabidopsis indole-3-acetic acid glucosyltransferase. J Biol Chem276: 4350–4356
Ji JH, Lee JS, Lee JS, Kim WT (1995) Effects of methyl jasmonate on ethylene production in tomato (Lycopersicon esculentum Mill.) hypocotyl segments and fruits. J Plant Biol38: 235–242
Kroczek RA, Siebert E (1990) Optimization of northern analysis by vacuum-blotting, RNA transfer, visualization and ultraviolet fixation. Anal Biochem184: 90–95
Lee H, Raskin I (1999) Purification, cloning, and expression of a pathogen inducible UDP glucose: Salicylic acid glucosyltransferase from tobacco. J Biol Chem274: 36637–36642
Li Y, Baldauf S, Lim E-K, Bowles DJ (2001) Phylogenetic analysis of the UDP glycosyltransferase multigene family ofArabidopsis thaliana. J Biol Chem276: 4338–4343
Normanly J (1997) Auxin metabolism. Physiol Plant100: 431–442
Sambrook J, Fritsch, EF, Maniatis T (1989) Molecular Cloning, A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, New York
Sembdner G, Atzorn R, Schneider G (1994) Plant hormone conjugation. Plant Mol Biol26: 1459–1481
Sembdner G, Parthier B (1993) The biochemistry and the physiological molecular actions of jasmonates. Annu Rev Plant Physiol Plant Mol Biol44: 569–589
Seo HS, Song JT, Cheong JJ, Lee YH, Lee YW, Hwang I, Lee JS, Choi YD (2001) Jasmonic acid carboxyl methyltransferase: A key enzyme for jasmonate-regulated plant responses. Proc Natl Acad Sci USA98: 4788–4793
Song JT, Seo HS, Song SI, Lee JS, Choi YD (2000) NTR1 encodes a floral nectary-specific gene inBrassica campestris L. ssp.pekinensis. Plant Mol Biol42: 647–655
Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine inArabidopsis. Plant Cell16: 2117–2127
Staswick PE, Tiryaki I, Rowe ML (2002) Jasmonate response locus JAR1 and several relatedArabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell14: 1405–1415
Szerszen JB, Szczyglowski K, Bandurski RS (1994)iaglu, a gene from Zeamays involved in conjugation of growth hormone indole-3-acetic acid. Science265: 1699–1701
Tarn YY, Epstein E, Normanly J (2000) Characterization of auxin conjugates inArabidopsis: Low steady-state levels of indole-3-acetyl-aspartate, indole-3-acetyl-glutamate, and indole-3-acetyl-glucose. Plant Physiol123: 589–596
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, J.T. Biochemical characterization of anArabidopsis glucosyltransferase with high activity toward Jasmonic acid. J. Plant Biol. 48, 422–428 (2005). https://doi.org/10.1007/BF03030584
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03030584