Abstract
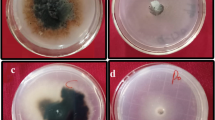
Verticillium dahliae Kleb. with a complicated genetic diversity is a widely distributed major pathogen resulting in cotton wilt, which causes high economic losses in cotton lint production in the cotton belt of Turkey. A collection of 70 TurkishV. dahliae isolates (68 from wilted cotton plants in 28 districts and two from watermelon plants in two districts) were tested for vegetative compatibility by observing heterokaryon formation among complementary nitrate-nonutilizing (nit) mutants. The mutants were tested against international reference tester isolates and also were paired with one another. Thirty-nine isolates were assigned to vegetative compatibility group (VCG) 2B, 19 to VCG2A and three to VCG4B. One isolate was self-incompatible and eight others could not be assigned to any of the identified VCGs because theirnit mutants showed negative reactions with the tester isolates of four VCGs or theirnit mutants reverted back to the wild type. This is the first report of VCGs inV. dahliae from cotton in Turkey.
Similar content being viewed by others
References
Bao, J.R., Katan, J., Shabi, E. and Katan, T. (1998) Vegetative compatibility groups inVerticillium dahliae from Israel.Eur. J. Plant Pathol. 104: 263–269.
Bicici, M. and Kurt, S. (1998) Etiology, incidence and prevalence of cotton wilt disease and strains of wilt pathogen in Cukurova.Proc. World Cotton Research Conf. 2 (Athens, Greece), pp. 914–918 (abstr.).
Correll, J.C., Klittich, C.J.R. and Leslie, J.F. (1987) Nitrate nonutilizing mutants ofFusarium oxysporum and their use in vegetative compatibility tests.Phytopathology 77: 1640–1646.
Daayf, F., Nicole, M. and Geiger, J.P. (1995) Differentiation ofVerticillium dahliae populations on the basis of vegetative compatibility and pathogenicity on cotton.Eur. J. Plant Pathol. 10: 69–79.
Elena, K. (1999) Genetic relationships amongVerticillium dahliae isolates from cotton in Greece based on vegetative compatibility.Eur. J. Plant Pathol. 105: 609–616.
Elena, K. and Paplomatas, E.J. (1998) Vegetative compatibility groups withinVerticillium dahliae isolates from different hosts in Greece.Plant Pathol. 47: 635–640. 7. Elena, K. and Tjamos, E.C. (1995) Vegetative compatibility groups ofFusarium oxysporum f.sp.dianthi from plants and the rhizosphere of carnation in Greece.Plant Pathol. 44:148-152.
Hawksworth, D.L. and Talboys, P.W. (1970)Verticillium dahliae. Descriptions of Pathogenic Fungi and Bacteria. C.M.I. No. 256.
Hiemstra, J.A. and Rataj-Guranowska, M. (2003) Vegetative compatibility groups inVerticillium dahliae isolates from the Netherlands as compared to VCG diversity in Europe and in the USA.Eur. J. Plant Pathol. 109: 827–839.
Joaquim, T.R. and Rowe, R.C. (1990) Reassessment of vegetative compatibility relationships among strains ofVerticillium dahliae using nitrate-nonutilizing mutants.Phytopathology 80: 1160–1166.
Katan, T. and Katan, J. (1988) Vegetative-compatibility grouping ofFusarium oxysporum f.sp.vasinfectum from tissue and the rhizosphere of cotton plants.Phytopathology 78: 852–855.
Korolev, N. and Katan, T. (1997) Improved medium for selecting nitrate nonutilizing (nit) mutants ofVerticillium dahliae.Phytopathology 87: 1067–1070.
Korolev, N., Katan, J. and Katan, T. (2000) Vegetative compatibility groups ofVerticillium dahliae in Israel: Their distribution and association with pathogenicity.Phytopathology 90: 529–536.
Korolev, N., Pérez-Artés, E., Bejarano-Alcázar, J., Rodríquez-Jurado, D., Katan, J., Katan, T.et al. (2001) Comparative study of genetic diversity and pathogenicity among populations ofVerticillium dahliae from cotton in Spain and Israel.Eur. J. Plant Pathol. 107: 443–456.
Kurt, S., Dervis, S. and Sahinler, S. (2003) Sensitivity ofVerticillium dahliae to prochloraz and prochloraz-manganese complex and control of Verticillium wilt of cotton in the field.Crop Prot. 23: 51–55.
Puhalla, J.E. (1979) Classification of isolates ofVerticillium dahliae based on heterokaryon incompatibility.Phytopathology 69: 1186–1189.
Strausbaugh, C.A., Schroth, M.N., Weinhold, A.R. and Hancock, J.G. (1992) Assessment of vegetative compatibility ofVerticillium dahliae tester strains and isolates from California potatoes.Phytopathology 82: 61–68.
Toth, K.F. and Lacy, M.L. (1991) Comparing vegetative compatibility and protein banding patterns for identification ofFusarium oxysporum f.sp.apii race 2.Can. J. Microbiol. 37: 669–674.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dervis, S., Bicici, M. Vegetative compatibility groups inVerticillium dahliae isolates from cotton in Turkey. Phytoparasitica 33, 157–168 (2005). https://doi.org/10.1007/BF03029975
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03029975