Abstract
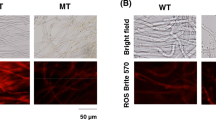
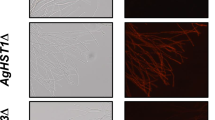
For this study, we hypothesized that mitochondrial NAD+-dependent isocitrate dehydrogenase 1 (ICDH1) and isocitrate lyase (ICL1) were important enzymes for riboflavin synthesis in the fungusAshbya gossypii. Here, the genes encoding ICDH1 and ICL1 were disrupted in order to analyze the enzymes' functions on riboflavin production by the fungus. The riboflavin production resulting from these disruptants was markedly decreased compared to the concentration produced by its parental strain when cultured in a rich nutrient medium used to optimize riboflavin production. Furthermore, when comparing the transcription levels of the genes encoding ICDH1 and ICL1, between wild-typeA. gossypii and an itaconate resistant mutant ofA. gossypii obtained by UV irradiation, the mRNA levels in the mutant were 1.8- and 2.0-fold higher than those in the wild-type strain, respectively. These results indicate that ICDH1 and ICL1 are key enzymes for riboflavin synthesis inA. gossypii.
Similar content being viewed by others
References
Stahmann, K. P., J. L. Revuelta, and H. Sculberger (2000) Three biotechnical processes usingAshbya gossypii, Candida famata, orBacillus subtilis compete with chemical riboflavin production.Appl. Microbiol. Biotechnol. 53: 509–516.
Demain, A. L. (1972) Riboflavin oversynthesis.Annu. Rev. Microbiol. 26: 369–388.
Dietrich, F. S., S. Voegeli, S. Brachat, A. Lerch, K. Gates, S. Steiner, C. Mohr, R. Pohlmann, P. Luedi, S. Choi, R. A. Wing, A. Flavier, T. D. Gaftney, and P. Philippsen (2004) TheAshbya gossypii geneme as a tool for mapping the ancientSaccharomyces cerevisiae genome.Science 304: 304–307.
Hermida, L., S. Brachat, S. Voegeli, P. Philippsen, and M. Primig (2005) The Ashbya Genome Database (AGD)—a tool for the yeast community and genome biologists.Nucleic Acids Res. 33: D348-D352.
Ming, H., A. V. Lara Pizarro, and E. Y. Park (2003) Application of waste activated bleaching earth containing rapeseed oil on riboflavin production in the culture ofAshbya gossypii.Biotechnol. Prog. 19: 410–417.
Park, E. Y. and H. Ming (2004) Oxidation of rapeseed oil in waste activated bleaching earth and its effect on riboflavin production in culture ofAshbya gossypii.J. Biosci. Bioeng. 97: 59–64.
Monschau, N., H. Sahm, and K. P. Stahmann (1998) Threonine aldolase overexpression plus threonine supplementation enhanced riboflavin production inAshbya gossypii.Appl. Environ. Microbiol. 64: 4283–4290.
Forster, C., M. A. Santos, S. Ruffert, R. Kramer, and J. L. Revuelta (1999) Physiological consequence of disruption of theVMAI gene in the riboflavin overproducerAshbya gossypii.J. Biol. Chem. 274: 9442–9448.
Schlupen, C., M. A. Santos, U. Weber, A. de Graaf, J. L. Revuelta, and K. P. Stahmann (2003) Disruption of theSHM2 gene, encoding one of two serine hydroxymethyl-transferase isoenzymes, reduces the flux from glycine to serine inAshbya gossypii.Biochem. J. 369: 263–273.
Kato, T. and E. Y. Park (2006) Expression of alamine: glyoxylate aminotransferase gene fromSaccharomyces cerevisiae inAshbya gossypii.Appl. Microbiol. Biotechnol. 71: 46–52.
Przybyla-Zawislak, B., D. M. Gadde, K. Ducharme, and M. T. McCammon (1999) Genetic and biochemical interactions involving tricarboxylic acid cycle (TCA) function using a collection of mutants defective in all TCA cycle genes.Genetics 152: 153–166.
Cupp, J. R. and L. McAlister-Henn (1991) NAD+-dependent isocitrate dehydrogenase.J. Biol. Chem. 266: 22199–22205.
Cupp, J. R. and L. McAlister-Henn (1992) Cloning and characterization of the gene encoding the IDH1 subunit of NAD+-dependent isocitrate dehydrogenase fromSaccharomyces cerevisiae.J. Biol. Chem. 267: 16417–16423.
Fernandez, E., F. Moreno, and R. Rodicio (1992) TheICLI gene fromSaccharomyces cerevisiae.Eur. J. Biochem. 204: 983–990.
Luttik, M. A., P. Kotter, F. A. Salomons, I. J. van der Klei, J. P. van Dijken, and J. T. Pronk (2000) TheSaccharomyces cerevisiae ICL2 gene encodes a mitochondrial 2-methylisocitrate lyase involved in propionyl-coenzyme A metabolism.J. Bacteriol. 182: 7007–7013.
Maeting, I., G. Schmidt, H. Sahm, J. L. Revuelta, Y. D. Stierhof, and K. P. Stahmann (1999) Isocitrate lyase ofAshbya gossypii—transcriptional regulation and peroxisomal localization.FEBS Lett. 444: 15–21.
Park, E. Y., J. H. Zhang, S. Tajima, and L. Dwiarti (2006) Isolation ofAshbya gossypii mutant for an improved riboflavin production targeting for biorefinery technology.J. Appl. Microbiol. In press.
Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (1992)Current Protocols in Molecular Biology, John Wiley & Sons Inc, Greene, NY, USA.
Sambrook, J., E. F. Fritsch, and T. Maniatis (1989)Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. USA.
Adams, A., D. E. Gottschling, C. A. Karser, and T. Stearns (1997)Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA.
Wendland, J., Y. Ayad-Durieux, P. Knechtle, C. Rebischung, and P. Philippsen (2000) PCR-based gene targeting in the filamentous fungusAshbya gossypii.Gene 242: 381–391.
Sauer, U., V. Hatzimanikatis, H. P. Hohmann, M. Manneberg, A. P. van Loon, and J. E. Bailey (1996) Physiology and metabolic fluxes of wild-type and riboflavin-producingBacillus subtilis.Appl. Environ. Microbiol. 62: 3687–3696.
Schmidt, G., K. P. Stahmann, and H. Sahm (1996) Inhibition of purified isocitrate lyase identified itaconate and oxalate as potential antimetabolites for the riboflavin overproducerAshbya gossypii.Microbiology 142: 411–417.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanamasa, S., Tajima, S. & Park, E.Y. Isocitrate dehydrogenase and isocitrate lyase are essential enzymes for riboflavin production inAshbya gossypii . Biotechnol. Bioprocess Eng. 12, 92–99 (2007). https://doi.org/10.1007/BF03028632
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03028632