Abstract
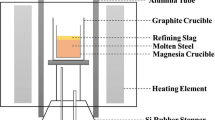
The correlation of the equilibrium behaviors of phosphorus and vanadium between slag and low carbon molten steel in inert atmosphere was investigated with respect to the experimental variables of slag basicity, the (P2O5) and (V2O5) content, and the reaction temperature. The distribution ratios of phosphorus and vanadium increased with an increase in the slag basicity. The logarithms of the vanadium distribution ratio were greater by a factor of about two than those of phosphorus in the range of low slag basicity, but the difference diminished with an increase in the slag basicity. The logarithms of the vanadium distribution ratio increased linearly with an increase in the (P2O5) and (V2O5) content in the slag, while those of phosphorus remained nearly constant. The logarithms of the phosphorus and vanadium distribution ratio decreased with an increase in temperature, and the dependence on temperature was greater for the phosphorus than for the vanadium. For both the maximization of the vanadium yield and the minimization of the rephosphorization of molten steel in the steelmaking process, the ratio of N(V2O5)/N(P2O5), the slag basicity, the ratio of f[P]/f[V], and the temperature should be maximized, and the (FeO) content in the slag should be minimized.
Similar content being viewed by others
References
A. B. Quisoe, S. F. Medina, J. M. Cabrera and J. M. Prado,Mat. Sci. Tech. 15, 635 (1999).
H. Adrian,Mat. Sci. Tech. 15, 366 (1999).
Liu Jie Xu, Shi Zhong Wei, Jian Dong Xing, Yan Li, and Long Ru,Met. Mater.-Int. 12, 371 (2006).
W. G. Jung,Technical Report, Kookmin University, Seoul (2000).
R. Inoue and H. Suito,Trans. ISIJ 22, 705 (1982).
H. Suito, R. Inoue, and M. Takada,Trans. ISIJ 21, 250 (1981).
J. J. Moore,Chemical Metallurgy, p. 152, Butterworths (1981).
B. D. You,Introduction of Steelmaking, p. 31, Inha University (2004).
The Japan Society for the Promotion of Science,Steelmaking data source book, p. 278 Gorden and Breach Science Publishers (1988).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, D.Y., Wee, C.H., Kim, M.S. et al. Distribution behavior of vanadium and phosphorus between slag and molten steel. Met. Mater. Int. 13, 171–176 (2007). https://doi.org/10.1007/BF03027569
Issue Date:
DOI: https://doi.org/10.1007/BF03027569