Abstract
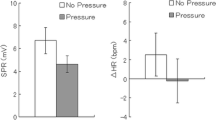
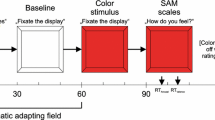
Several recent studies have indicated that the degree and direction of bilateral skin conductance (BSC) asymmetry and the direction of conjugate lateral eye movements (CLEMs) both index hemispheric functioning. Evidence also suggests that BSC asymmetry decreases and CLEMs become more unidirectional as level of arousal increases. Thirty-two normal adults received an habituation series of five-second tones followed by 40 questions. All stimuli were tape recorded. The questions (Schwartz, Davidson, and Maer, 1975) were grouped in four series: 10 verbal-emotional, 10 verbalnonemotional, 10 spatial-emotional, and 10 spatial-nonemotional. The order in which the four series were presented was counterbalanced across subjects. Prior to each question, subjects fixated the face of a mannequin. Sixteen subjects (high arousal) had five seconds to contemplate their answer; the remaining 16 (low arousal) had 10 seconds. Instructions for the high arousal group specified that intelligence and problem-solving ability was being tested, and that speed and accuracy were very important. Those for the low arousal group indicated casually that subjects were to ponder a series of questions. BSC levels, SC response amplitudes, and CLEMs were measured during a five-second interval following each tone or question. BSC levels were greater under the High Arousal condition. While approximately two-thirds of the subjects showed BSC asymmetry (roughly evenly divided between those with larger left and right hand responses), neither the degree nor direction of the asymmetries changed as a function of question type. While CLEM direction was more sensitive to question type under low arousal, (i.e., verbal questions resulted in predominantly right-going CLEMs and spatial questions resulted in predominantly left-going CLEMs) they tended to be stereotyped (i.e., unidirectional) under high arousal. Finally, no relationship was observed between CLEM direction and BSC asymmetry under either high or low arousal condition.
Similar content being viewed by others
References
Bakan, P.: Hypnotizability, laterality of eye movements, and functional brain asymmetry. Perceptual and Motor Skills,28, 927–932, 1969.
Day, M. E.: An eye-movement phenomena relating to attention, thought and anxiety. Perceptual and Motor Skills.19, 443–446, 1964.
Fisher, S.: Body image and asymmetry of body reactivity. The Journal of Abnormal and Social Psychology57, 292–298, 1958.
Galin, D.: Implications for psychiatry of left and right cerebral specialization. Archives of General Psychiatry31, 572, 1974.
Galin, D. and Ornstein, R.: Lateral specialization of cognitive modes: I an EEG study. Psychophysiology9, 412–418, 1972.
Galin, D., and Ornstein, R.: Individual differences in cognitive style: reflective eye movements. Neuropsychologia12, 367–376, 1974.
Gazzaniga, M. S., Bogen, J. E. and Sperry, R. W.: Some functional effects of sectioning the cerebral hemispheres in man. Proceedings of the National Academy of Science48, 1765, 1962.
Gazzaniga, M. S., Bogen, J. E. and Sperry, R. W.: Laterality effect in somethesis following cerebral commisurotomy in man. Neuropsychologia1, 209, 1963.
Gazzaniga, M. S., Bogen, M. E. and Sperry, R. W.: Observations on visual perception after disconexion of the cerebral hemispheres in man. Brain688, 221–236, 1965.
Gruzelier, J. H.: Bilateral asymmetry of skin conductance orienting activity and levels in schizophrenics. Journal of Biological Psychology1, 21–41, 1973.
Gruzelier, J. H.: Bimodal arousal and lateralized dysfunctions in schizophrenics: the effects of chlorpromazine upon physiological information processing and endocrine measures.In: L. Wynne, R. Cromwell and S. Mathses (eds.).Nature of Schizophrenia: New Findings and Future Strategies, New York, John Wiley and Sons, 1977.
Gruzelier, J. H. and Hammond, N.: Schizophrenia: a dominant hemisphere temporal-limbic disorder? Research Communications in Psychology, Psychiatry, and Behavior1, 33–72, 1976.
Gruzelier, J. H. and Venables, P. H.: Skin conductance orienting activity in a heterogeneous sample of schizophrenics. The Journal of Nervous and Mental Disease155, 277–287, 1972.
Gruzelier, J. H. and Venables, P. H.: Skin conductance responses to tones with and without attentional significance in schizophrenic and non-schizophrenic psychiatric patients. Neuropsychologia2, 221–230, 1973.
Gur, R. E. and Gur, R. C.: Defense mechanisms, psychosomatic symptomotology, and conjugate lateral eye movements. Journal of Consulting and Clinical Psychology43, 416–420, 1975.
Gur, R. E. and Gur, R. C.: Correlates of conjugate lateral eye movements.In: S. Hamad, R. Doty, L. Goldstein, J. Joynes and G. Krauthamer (eds.),Lateralization in the Nervous System, New York, Academic Press, 1977.
Gur, R. E., Gur, R. C. and Harris, L. J.: Cerebral activation, as measured by subjects; lateral eye movements, is influenced by experimenter location. Neuropsychologia13, 35–44, 1975.
Hamad, S. R.: Creativity, lateral saccades, and the nondominant hemisphere. Perceptual and Motor Skills34, 653–654, 1972.
Holloway, F. A. and Parsons, A.: Unilateral brain damage and bilateral skin conductance levels in humans. Psychophysiology6, 138–148, 1969.
Kinsbourne, M.: Eye and head turning indicates cerebral lateralization. Science176, 539–541, 1972.
Kocel, K., Galin, D., Ornstein, R. and Merrin, E.: Lateral eye movements and cognitive mode. Psychonomic Science27, 223–224, 1972.
Lacroix, J. M. and Comper, P.: Lateralization in the electrodermal system as a function of cognitive/ hemispheric manipulations. Psychophysiology16, 116–129, 1979.
Myslobodsky, M. S. and Hoersch, N.: Bilateral electrodermal activity in depressive patients. Unpublished manuscript, Tel-Aviv University, 1977.
Myslobodsky, M. and Rattok, J.: Asymmetry of electrodermal activity in man. Bulletin of the Psychonomic Society6, 501–502, 1975.
Myslobodsky, M. and Rattok, J.: Bilateral electrodermal activity in waking man. Acta Psychologia41, 273–282, 1977.
Obrist, P. A.: Skin resistance levels and galvanic skin response; unilateral differences. Science139, 227–228, 1963.
Schwartz, G. E. Davidson, R. J. and Maer, F.: Right hemisphere lateralization for emotion in the human brain: interactions with cognition. Science190, 286–288, 1975.
Weitan, W. and Etaugh, C.: Lateral eye-movements as related to verbal and perceptual-motor skills, and values. Perceptual and Motor Skills36, 423–428, 1973.
Wyatt, R. and Tursky, B.: Skin potentials in rightand left-handed males. Psychophysiology6, 133–137, 1969.
Author information
Authors and Affiliations
Additional information
These data were submitted in partial fulfillment of the requirements for the MA degree to the Department of Psychology, Southern Illinois University at Edwardsville. Present address: Department of Psychology, Southern Illionois University at Carbondale.
Supported by a grant to K. M. Kleinman from the Graduate School of Southern Illinois University, Edwardsville, Illinois.
Rights and permissions
About this article
Cite this article
Erwin, R.J., McClanahan, B.A. & Kleinman, K.M. Effects of level of arousal and type of task on bilateral skin conductance asymmetry and conjugate lateral eye movements. Pav. J. Biol. Sci. 15, 59–67 (1980). https://doi.org/10.1007/BF03003684
Issue Date:
DOI: https://doi.org/10.1007/BF03003684