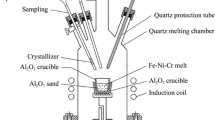
Abstract
The hydrogen content in liquid binary aluminum alloys with 1, 3, 5and 8wt% iron has been determined in the temperature range from 973K to 1103K. The hydrogen content in molten Al-Fe alloys increases remarkably when the temperature of the melt rises to about 1053K. This work indicates that the alloying element iron plays an important role in hydrogen content in superheated Al-Fe alloy melts below about 1053K. The results make it clear that the hydrogen content in the melt aluminum reduces with the increasing element levels. A conclusion is drawn that the degree of gassing in molten Al-Fe alloys is bound up with the properties of oxide film of aluminum alloy melts. The element iron has no effect on the compact structure of oxide film in aluminum melts. The effects of alloying element are theoretically analyzed in terms of Wagner interaction parameter. According to the values of the first order interaction parameter, it is concluded that the interaction between iron atom and aluminum is much stronger than that between hydrogen atom and aluminum, and the addition of the alloying element decreases the affinity of liquid aluminum for hydrogen.
Similar content being viewed by others
References
Bauhloh W and Bedjali M. Influence of Alloy Element on Hydrogen Content in Aluminum Melt.Metallwirtschaft, 1942, 21: 683–686
Baukloh W and Oesterlen F. Hydrogen Content in Aluminum-silicon Alloys.Ztsch. Metallkunde, 1938, 30: 386–390
LI Xi-zhenet al, Infleunce of Element Cu on Hydrogen Content in Superheated Aluminum Melt.Trans. Nonferrous Met. Soc. China, 2001, 11: 358–360
Opie W R and Grant N J. Hydrogen Solubility in Aluminum and Some Aluminum Alloys.Transactions AIME, 1950, 188: 1237–1241
Anyalebechi P N Talbot D E J, and Granger D A. The Solubility of Hydrogen in Liquid Binary Al-Li Alloys.Met. Trans. B, 1988, 19B: 227–232
D Stephensoon.Forecasting Theory on Hydrogen Behavior in Liquid Aluminum Alloys [Ph. D. Thesis]. Brunel University, Middlesex, England: Brunel University Press, 1978
Grusleski J E.The Treatment of Liquid Aluminum-Silicon Alloys.AFS Inc. Des Plaines III, 1990: 143–168
Pendersen T. Refining Efficiency on Hydrogen, Alkaline Metals and Inclusions in the Hydro Metal Refining System.Light Metals, TMS, Edited by Elwin L Rooy 1991, 1063–1067
Talbot D E J and Anyalebechi P N. Solubility of Hydrogen in Liquid Aluminum.Materials Science and Technology, 1988, 4: 1–4
Ransley C E, Talbot D E J. The Solubility in Aluminum and Some Aluminum Alloys.Tram AIME, 1950, 188: 1237–1241
Zhonghua Z, Xiufang B,et al. Hydrogen Content and Structure of Aluminum Melt.Acta Metallurgica Sinica, 2000, 36: 33–36
Prince N Anyalebechi. Analysis of the Effects of Alloying Elements on Hydrogen Solubility in Liquid Aluminum Alloys.Scripta Metallurgica et Material, 1995, 33: 1209–1216
Wagner C.Thermodynamics of Alloys. New York: Addison-Wesley, 1962: 51
J Chipman. Thermodynamics of Alloy System.J. Iron and Steel, 1955, 180: 97–103
C H P Lupis and J F Elliott. The Microdynamics Behavior of Aluminum Alloys.Acta Met., 1966, 14: 526–531
H Shahani.Influence of Electric Concentration on Solubility of Hydrogen in Alloys [Ph. D. Thesis]. The Royal Institute of Technology, Stockholm, Sweden: Metallurgical Industry Press, 1984
Zuyao X.Thermodynamics of Metal Materials. Pekin: Science Publishing Company, 1981: 112
Jingyu, Q, Xiufang B. Micro-inhomogeneous Structure of Liquid Al-Fe Alloys.Science in China, 1998, 28: 97–101
Author information
Authors and Affiliations
Corresponding author
Additional information
Founded by the the National Natural Science Foundation of China, (Grant No. 50071028) and the Natural Science Foundation of Shandong (No. Z2001F02).
Rights and permissions
About this article
Cite this article
Hu, Ln., Bian, Xf., Ananda, M. et al. Influence of elemental iron on hydrogen content in superheated aluminum-iron melts. Journal of Wuhan University of Technology-Mater. Sci. Ed. 19, 57–61 (2004). https://doi.org/10.1007/BF03000170
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03000170