Abstract
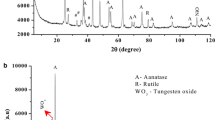
Ammonium metatungstate and cobalt nitrate were mixed at the molecular level in distilled water and then spray-decomposed to CoWO4/WO3 nanocomposite powder. The particle morphology, crystalline size, forming course, chemical composition and phase structure of the powder were studied by SEM, TEM, DTA-TG, IR and XRD, respectively. Results show that the powder is homogeneous, spherical and nano-aggregated.
Similar content being viewed by others
References
K J A Brookes. News: Iard Materials at Euro PM 2002.Int ’ l. J. Ref. Metals & Hard Mater., 2003, 21: 81–103
Z Y Zhang, M Muhammed. Thermochemical Decomposition of Cobalt Doped Ammonium Paratungstate Precursor.Thermochim Acta, 2003, 400(1-2): 235–245
G Gille, B Szesny, K Dreyer, H van den Berg, J Schmidt, T Gestrich, G. Leitner. Submicron and Ultrafine Grained Hardmetals for Microdrills and Metal Cutting Inserts.Int’ l. J. Ref. Metals & Hard Mater., 2002, 20: 3–22
E Lassner, W-D Schubert.Tungsten-Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds. London, UK: Kluwer Academic/Plenum Publishers, 2000
G S Upadhyaya.Cemented Tungsten Carbides —Production, Properties, and Testing. New Jersey, USA: Noyes Publications, Westwood, 1998
B-K Kim, G-G Lee, G-H Ha, D-W Lee.Mechanochemical Process for Producing Fine WC/ Co Composite Powder. US Pat.: No. 5882376, 25 July, 1997
M Sherif El-Eskandarany, A A Mahday, H A Ahmed, A H Amer. Synthesis and Characterizations of Ball-Milled Nanocrystalline WC and Nanocomposite WC-Co Powders and Subsequent Consolidations.Journal of Alloys and Compounds, 2000, 312: 315–325
Y T Zhu, A Manthiram. A New Route for the Synthesis of Tungsten Carbide-Cobalt Nanocomposite.J. Am. Ceram. Soc., 1990, 77(10): 2777–2778
B V Krishna, K V Gaganpreet, H Bhunia. Synthesis of WC-Co Nanocomposites by Using Polymers asin situ Carbon Source.Int’ l. J. Nanoscience, 2002, 1(2): 139–148
Z Y Zhang, S Wahlberg, M S Wang. Processing of Nanostructured WC-Co Powder from Precursor Obtained by Co-precipitation.NanoStruct. Mater., 1999, 12(1-4): 163–166
Z-G Ban, L L Shaw. Synthesis and Processing of Nanostructured WC-Co Materials.J. Mater. Sci., 2002, 37: 3397–3403
L E McCandlish, B H Kear, S-J Bhatia.Spray Conversion Process for the Production of Nanophase Composite Powders. World Pat.: W0 91/07244,30 May, 1991
L E McCandlish, B H Kear, B K Kim.Carbothermic Reaction Process for Making Nanophase WC-Co Powder. World Pat.:W0 93/02962, 18 Feb., 1993
G Q Shao, B L Wu, X L Duan, J R Xie, M K Wei, R Z Yuan.WC-Co Nanocrystalline Composite Powder without η Phases Produced on an Industrial Scale. Chinese Invention Pat.: ZL 99 1 16597.7, 13 Aug., 1999
G Q Shao, B L Wu, X L Duan, J R Xie, M K Wei, R Z Yuan. Low Temperature Carbonization of W-Co Salts Powder. In: E Ustundag and G Fishman eds.,Ceramic Engineering & Science Proceedings- 23rdAnnual Conference on Composites, Advanced Ceramics, Materials, and Structures: A, Ohio, USA: The American Ceramic Society, 1999: 45–50
G Q Shao, B L Wu, X L Duan, J R Xie, M K Wei, R Z Yuan. Nanocrystalline Grains & Superfine Particles of Tungsten Carbide-Cobalt Powders. In: N P Bansal and J P Singh eds.,Innovative Processing/ Synthesis: Ceramics, Glasses, Composites IV, Ohio, USA: The American Ceramic Society, 2000: 375–383
G Q Shao, X L Duan, B L Wu, J R Xie, M K Wei, R Z Yuan. Continuous Reduction Carburisation Mechanism of Precursor-Derived Nanocrystalline WC-Co. In: J P Singh, NP Bansal and E Ustundag eds.,Advances in Ceramic Matrix Composites VI, Ohio, USA: The American Ceramic Society, 2000: 207–217
G Q Shao, X L Duan, J R Xie, F Zhang, B L Wu, R Z Yuan. Forming & Controlling of WC-Co Nonmetal-Metal Nano-Composite Structure.J. Chin. Ceram. Soc. (in Chinese), 2002, 30(1): 40–44
G Q Shao, B L Wu, M K Wei, R Z Yuan. Developments of WC Hardmetals with Ultrafine Grain Size.J. Wuhan Univ. Technol. (in Chinese), 1999, 21 (6): 18–20
G Q Shao, B L Wu, X L Duan, J R Xie, M K Wei, R Z Yuan. Preparation of WC-Co Powder by the Fluidization Technology.ACTA Metall. Sinica (in Chinese), 1999, 35(2): 144–146
B L Wu, G Q Shao, X L Duan, J R Xie, M K Wei, R Z Yuan. Nanocrystalline Composite Powder of WC-Co Produced on an Industrial Scale.Mater. Rev. (in Chinese), 2000, 14(7): 55–58
G Q Snao, X L Duan, J R Xie, X H Yu, W F Zhang, R Z Yuan. Sintering of Nanocrystalline WC-Co Composite Powder.Reviews on Advanced Materials Science, 2003, 5(4): 281–286
R J Zeng, B Rand. Comparison of Various Particle Sizing Techniques.J. Wuhan Univ. Technol.-Mater. Sci. Ed., 2000, 15 (2): 7–14
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by High Technology Research and Development Programme of China (Grant: 2002AA302504), the Key Project of Hubei Province, China (Grant: 2001AA101B03), and the National Natural Science Foundation of China(Grant:50220160657).
Rights and permissions
About this article
Cite this article
Shao, Gq., Guo, Jk., Xie, Jr. et al. The preparation of CoWO4/WO3 nanocomposite powder. Journal of Wuhan University of Technology-Mater. Sci. Ed. 19, 1–3 (2004). https://doi.org/10.1007/BF03000154
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03000154