Abstract
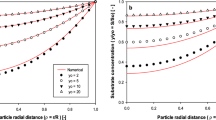
The determination of kinetic parameters such as the apparent Michaelis constant (Km app.) of enzymic reactions occurring at a mixed interface, that is, at a solid/liquid interface where the catalyst is bound to the solid phase, can lead to values that are too high when an unstirred layer is present. The perturbatior, for which an equation is derived in this paper, depends on the thickness of the unstirred layer, the maximal catalytic rate, and the diffusion constant. An additional error is introduced by the graphical determination of Km using the Lineweaver-Burk plot since after transformation the relation is not linear if an unstirred layer is present. A numerical example shows that apparent Michaelis constants obtained from in vitro experiments involving immobilized enzymes can lead to misleading results originating from conditions used in experiments such as stirring rate (CSTR-continuous stirred tank reactors) or flow rate (packed-bed reactors) of substrate. This can partly be explained by a greater bias of the Km determination caused by unstirred layers of the liquid medium (substrate) being present around the solid particles onto which the enzyme molecules are attached.
Similar content being viewed by others
References
Laidler, K. J., andSundaram, P. V. (1971)In Chemistry of the Cell Interface, Part A, Academic Press, New York, p. 317.
Sundaram, P. V. (1974)In Proceedings of the 2nd Enzyme Engineering conference,Pye, E. K. (ed.), Plenum Press, New York, p. 283.
Sundaram, P. V., Tweedale, A., andLaidler, K. J. (1970) Can. J. Chem. 48:1498.
Sundaram, P. V. (1973) Biochim. Biophys. Acta 321: 319.
Sundaram, P. V., andJoy, K. W. (1978) J. Solid-Phase Biochem. 3: 223.
Miller, D. M. (1972) Biochim. Biophys. Acta 266: 85.
Shàafi, R. I., Rich, G. T., Sidel, V. W., Bossert, W., andSolomon, A. K. (1967) J. Gen. Physiol. 50: 1377.
Beck, R. E., andSchultz, J. S. (1972) Biochim. Biophys. Acta 255: 273.
Andreoli, T. E., Dennis, V. W., andWeigl, A. M. (1969) J. Gen. Physiol. 53: 133.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sundaram, P.V. An analysis and interpretation of the Michaelis-menten kinetics of immobilized enzymes perturbed by film diffusion. Journal of Solid Phase Biochemistry 3, 241–246 (1978). https://doi.org/10.1007/BF02991850
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02991850