Abstract
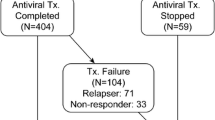
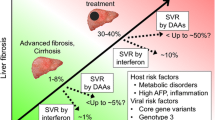
Hepatitis C virus (HCV) infection is responsible for more than a half of the cases of chronic viral hepatitis in Japan. About 20% of patients who are chronically infected with the virus develop cirrhosis about 20–30 years after the infection, with hepatocellular carcinoma developing in about 5% of patients a year. The only drug that effectively reduces the virus is interferon, but complete eradication of the virus can be obtained in only 30%–40% of treated patients. Reevaluation of the predictive factors to eradicate the virus by 24-week interferon therapy showed that the genotype other than 1b, a low virus load, and multiple amino acid substitutions in the interferon sensitivity determining region (ISDR) of genotype 1b are statistically significant predictive factors. Amino acid substitution in the PePHD domain of the E2 protein was rare and was unrelated with the outcome of interferon therapy. The fluctuation of the virus titer measured by branched DNA during a 2-year observation period was less that 10-fold in most patients, and amino acid substitutions in the ISDR were rare in such patients, suggesting that one point measurement of these parameters may be useful to select candidates for interferon therapy. A comparison of patients treated with interferon and untreated patients from the viewpoint of cancer prevention showed only a slight decrease in the risk of treated patients developing hepatocellular carcinoma. However, patients who showed normal alanine amino-transaminase (ALT) irrespective of virus clearance showed a significantly reduced risk of liver carcinogenesis. Similarly, a retrospective study to evaluate the long-term preventive effect of glycyrrhizin on hepatocellular carcinoma development showed that the therapy was effective in lowering the ALT value and in preventing liver carcinogenesis.
Similar content being viewed by others
References
Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 1993; 328: 1797–1801.
Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis-a prospective observation of 2215 patients. J Hepatol 1998; 28: 930–8.
Niederau C, Lange S, Heintges T, Erhardt A, Buschkamp M, Hurter D, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology 1998; 28: 1687–95.
Dusheiko GM. The natural course of chronic hepatitis C: implications for clinical practice. J Viral Hepat 1998; 5 (suppl 1): 9–12.
Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin: Irish Hepatology Research Group. N Engl J Med 1999; 340: 1228–33.
Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC Jr, Perrillo RP, et al. Treatment of Chronic hepatitis C with recombinant interferon alfa. N Engl J Med 1989; 321: 1501–6.
Di Bisceglie AM, Martin P, Kassianides C, Lisker-Melman M, Murray L, Waggoner J, et al. Recombinant interferon alfa therapy for chronic hepatitis C. N Engl J Med 1989; 321: 1506–11.
Chayama K, Saitoh S, Arase Y, Ikeda K, Matsumoto T, Sakai Y, et al. Effect of interferon administration on serum hepatitis C virus RNA in patients with chronic hepatitis C. Hepatology 1991; 3: 1040–3.
Shindo M, Di Bisceglie AM, Hoofnagle JH. Long-term follow-up of patients with chronic hepatitis C treated with interferon. Hepatology 1992; 15: 1013–6.
Enomoto N, Sakuma I, Asahina Y, Kurosaki M, Murakami T, Yamamoto C, et al. Comparison of full-length sequences of interferon-sensitive and resistant hepatitis C virus 1b. J Clin Invest 1995; 96: 224–30.
Enomoto N, Sakuma I, Asahina Y, Kurosaki M, Murakami T, Yamamoto C, et al. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus lb infection. N Engl J Med 1996; 334: 77–81.
Chayama K, Tsubota A, Kobayashi M, Okamoto K, Hashimoto M, Miyano Y, et al. Pretreatment virus load and multiple amino acid substitutions in the interferon sensitivity-determining region predict the outcome of interferon treatment in patients with chronic genotype 1b hepatitis C virus infection. Hepatology 1997; 25: 745–9.
Murashima S, Ide T, Miyajima I, Kumashiro R, Ueno T, Sakisaka S, et al. Mutations in the NS5A gene predict response to interferon therapy in Japanese patients with chronic hepatitis C and cirrhosis. Scand J Infect Dis 1999; 31: 27–32.
Nakano I, Fukuda Y, Katano Y, Nakano S, Kumada T, Hayakawa T. Why is the interferon sensitivity-determining region (ISDR) system useful in Japan? J Hepatol 1999; 30: 1014–22.
Sakuma I, Enomoto N, Kurosaki M, Izumi N, Marumo F, Sato C. Differential effect of interferon on hepatitis C virus 1b quasispecies in the nonstructural protein 5A gene. J Infect Dis 1999; 180: 1001–9.
McKechnie VM, Mills PR, McCruden EA. The NS5a gene of hepatitis C virus in patients treated with interferon-alpha. J Med Virol 2000; 60: 367–78.
Paterson M, Laxton CD, Thomas HC, Ackrill AM, Foster GR. Hepatitis C virus NS5A protein inhibits interferon antiviral activity, but the effects do not correlate with clinical response. Gastroenterology 1999; 117: 1187–97.
Squadrito G, Orlando ME, Cacciola I, Rumi MG, Artini M, Picciotto A, et al. Long-term response to interferon alpha is unrelated to “Interferon sensitivity determining region” variability in patients with chronic hepatitis C virus-1b infection. J Hepatol 1999; 30: 1023–7.
McKechnie VM, Mills PR, McCruden EA. The NS5a gene of hepatitis C virus in patients treated with interferon-alpha. J Med Virol 2000; 60: 367–78.
Hofgartner WT, Polyak SJ, Sullivan DG, Carithers RL Jr, Cretch DR. Mutations in the NS5A gene of hepatitis C virus in North American patients infected with HCV genotype 1a or 1b. J Med Virol. 1997; 53: 118–26.
Khorsi H, Castelain S, Wyseur A, Izopet J, Canva V, Rombout A, et al. Mutations of hepatitis C virus 1b NS5A 2209–2248 amino acid sequence do not predict the response to recombinant interferon-alfa therapy in French patients. J Hepatol 1997; 27: 72–7.
Chung RT, Monto A, Dienstag JL, Kaplan LM. Mutations in the NS5A region do not predict interferon-responsiveness in American patients infected with genotype 1b hepatitis C virus. J Med Virol. 1999; 58: 353–8.
Squadrito G, Leone F, Sartori M, Nalpas B, Berthelot P, Raimondo G, et al. Mutations in the nonstructural 5A region of hepatitis C virus and response of chronic hepatitis C to interferon alfa. Gastroenterology 1997; 113: 567–72.
Zeuzem S, Lee JH, Roth WK. Mutations in the nonstructural 5A gene of European hepatitis C virus isolates and response to interferon alfa. Hepatology 1997; 25: 740–4.
Taylor DR, Shi ST, Romano PR, Barber GN, Lai MM. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science 1999; 285: 107–10.
Chayama K, Tsubota A, Arase Y, Saitoh S, Koida I, Ikeda K, et al. Genotypic subtyping of hepatitis C virus. J Gastroenterol Hepatol 1993; 8: 150–6.
Detmer J, Lagier R, Flynn J, Zayati C, Kolberg J, Collins M, et al. Accurate quantification of hepatitis C virus (HCV) RNA from all HCV genotypes by using branched-DNA technology. J Clin Microbiol 1996; 34: 901–7.
Kwok S, Higuchi R. Avoiding false positive with PCR. Nature 1989; 339: 237–8.
SPSS Base System Syntax Reference Guide. Release 6.0. SPSS, 1993.
Davis GL. Prediction of response to interferon treatment of chronic hepatitis C. J Hepatol 1994; 21: 1–3.
Tsubota A, Chayama K, Ikeda K, Arase Y, Koida I, Saitoh S, et al. Factors predictive of response to interferon-alpha therapy in hepatitis C virus infection. Hepatology 1994; 19: 1088–94.
Yoshioka K, Kakumu S, Wakita T, Ishikawa T, Itoh Y, Takayanagi M, et al. Detection of hepatitis C virus by polymerase chain reaction and response to interferon-alpha therapy: relationship to genotypes of hepatitis C virus. Hepatology 1992; 16: 293–9.
Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, et al. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 1997;79: 1494–500.