Abstract
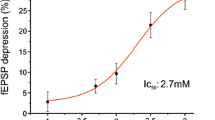
The involvement of the cyclic AMP (cAMP) effector system in the release of endogenous dopamine and acetylcholine from the rat neostriatum was assessed. Forskolin, an activator of adenylate cyclase, was used to enhance cAMP production, and the consequence of this enhancement on the spontaneous and potassium stimulated release of dopamine and a-cetylcholine was evaluated. Neostriatal slices were prepared from Fischer 344 rats and after a preincubation period the release of each endogenous neurotransmitter was measured from the same slice preparation. To measure acetylcholine release the slice acetylcholinesterase (AChE) activity was inhibited with physostigmine, but the release from slices with intact AChE activity was also determined (choline, instead of acetylcholine was detected in the medium). Under both conditions forskolin induced a significant dose-dependent increase in the potassium-evoked release of dopamine. In the same tissue preparations the release of neither acetylcholine (AChE inhibited) nor choline (AChE intact) was affected by forskolin. The results indicate that the cAMP second messenger system might be involved in neuronal mechanisms that enhance neostriatal dopamine release, but stimulation of this second messenger by forskolin does not further enhance neostriatal acetylcholine release.
Similar content being viewed by others
References Cited
Baizer, L. and Weiner, N., Nerve greowth factor treatment enhances nicotine-stimulated dopamine release and increases in cyclic adenosine 3′∶5′-monophosphate levels in PC 12 cell cultures.J. Neurosci., 5, 1176–179 (1985).
Beani, L., Bianchi, C., Siniscalchi, A., Sivilotti, L., Tanganelli, S. and Veratti, E., Different approaches to study acetylcholine release: endogenous ACh verses tritium efflux.Naunyn Schmiedeberg's Arch. Pharmacol., 328, 119–126 (1984).
Bowyer, J.F. and Weiner, N., K+-channel and adenylate cyclase involvement in regulation of Ca++-evoked release of [3H]dopamine from synaptosomes.J. Pharmacol. Exp. Ther., 248, 514–520 (1989).
Briggs, C. A., McAfee, D. A. and McCaman, R. E., Longterm regulation of synaptic acetylcholine release and nicotinic transmission.Br. J. Pharmacol., 93, 399–411 (1988).
Cubeddu, L. X. and Hoffman, I. S., Frequency-dependent release of acetylcholine and dopamine from rabbit striatum: its modulation by dopaminergic receptors.J. Neurochem., 41, 94–101 (1983).
Dryden, W. F., Singh, Y. N., Gordon, T. and Lazarenko, G., Pharmacological elevation of cAMP and transmitter release at the mouse neuromuscular junction.Can. J. Physiol. Pharmacol., 66, 207–212 (1987).
Freeman, J. J., Choi, R. L. and Jenden, D. J., Plasma choline, its turnover and exchange with brain choline.J. Neurochem., 24, 729–734 (1975).
Herdon, H., Strupish, J. and Nahorski, S. R., Differences between the release of radiolabelled and endogenous dopamine from superfused rat brain slices: Effects of depolizing stimuli, amphetamine and synthesis inhibition.Brain Res., 348: 309–320 (1985)
Jenden, D. J., Roch, M. and Booth, R., Simultaneous measurement of endogenous and deuterium labeled tracer variants of choline and acetylcholine in subpicomole quantities by gas chromatography mass spectrometry.Anal. Biochem., 55, 438–448 (1973).
Lee, H.-J., Alcorn, L. M. and Weiler, M. H., Effects of various experimental manipulation on neostriatal a-cetylcholine and dopamine release.Neurochemical Res., 16, 875–883 (1991).
Lehman, J. and Langer, S. Z., Muscarinic receptors on dopamine terminals in the cat caudate nucleus: Neuromodulation of [3H]dopamine release in vitro by endogenous acetylcholine.Brain Res., 248, 61–69 (1982).
Lehman, J. and Langer, S. Z., The striatal cholinergic interaction: synaptic target of dopaminergic terminals?Neurosci., 10, 1105–1120 (1983).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J., Protein measurement with the Folin reagent.J. Biol. Chem., 193, 265–275 (1951).
Markstein, R., Digges, K., Marshall, N. R. and Starke, K., Forskolin and the release of noradrenaline in cerebrocortical slices.Naunyn Schmeideberg's Arch. Pharmacol., 325, 17–24 (1984).
Masserano, J. M. and Weiner, N., Tyrosine hydroxylase regulation in the central nervous system.Mol. Cell. Biochem., 53/54, 129–152 (1983).
McIlwain, H. and Rodnight, R., Preparing neural tissues for metabolic studyin vitro. In McIlwain, H. (Eds.),Practical Neurochemistry, Churchill, London, pp. 109–188, 1962.
Patrick, R. L. and Barchas, J. D., Dopamine synthesis in rat brain striatal synaptosomes. II. Dibutyryl cyclic adenosine 3′∶5′-monophosphoric acid and 6-methyltetrahydropteridine-induced synthesis increase without an increase in endogenous dopamine release.J. Pharmacol. Exp. Ther. 197, 97–104 (1976).
Reese, J. H. and Cooper, J. R., Stimulation of a-cetylcholine release from Guinea-pig ileal synaptosomes by cyclic nucleotides and forskolin. Biochemical Pharmacol., 33, 3007–3011, (1984).
Seamon, K. B. and Daly, J. W., Forskolin, cyclic AMP and cellular physiology.Trends Pharmacol. Sci., 4, 120–123 (1983).
Seamon, K. B., Padgett, W. and Daly, J. W., Forskolin: Unique diterpene activator of adenylate cyclase in membranes and in intact cells.Proc. Natl. Acad. Sci. USA, 78, 3363–3367 (1981).
Stoof, J. C. and Kebabian, K. W., Independentin vitro regulation by D2-dopamine receptor of dopamine-stimulated efflux of cAMP and K+-stimulated release of acetylcholine from rat neostriatum.Brain Res., 250, 263–270 (1982).
Tsujimoto, A., Morita, K., Kitayama, S. and Dohi, T., Facilitation of acetylcholine-evoked CA release by cAMP on isolated perfused dog adrenal glands.Arch. Int. Pharmacodyn. Ther., 279, 304–313 (1986).
Weiler, M. H., Muscarinic modulation of endogenous acetylcholine release in rat neostriatal slices. J. Pharm. Exp. Ther. 250, 617–623 (1989).
Weiss, S., Forskolin attenuates the evoked release of [3H]GABA from s striatal neurons in primary culture.Brain Res., 463, 182–186 (1988).
Yau, W. M., Dorsett, J. A. and Youther, M. L., Stimulation of acetylcholine release from myenteric neurons of Guinea pig small intestine by forskolin.J. Pharmacol. Exp. Ther., 243, 507–510 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, HJ. Effects of forskolin on endogenous dopamine and acetylcholine release in rat neostriatal slices. Arch. Pharm. Res. 19, 520–528 (1996). https://doi.org/10.1007/BF02986022
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02986022