Abstract
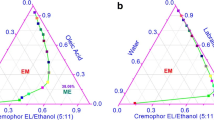
To improve the skin permeability of piroxicam, a new oil-in-water microemulsion containing 0.5% piroxicam was developed. Among various oils investigated for their suitability as an oil phase for the microemulsion system, oleic acid showed both excellent solubility and skin permeation enhancing effect for piroxicam. Microemulsion existence ranges were identified through the construction of the pseudo-ternary phase diagram. The effect of the content of oleic acid and the ratio of the surfactant/cosurfactant on skin permeation of piroxicam were evaluated with excised rat skins. The optimum formulation with the highest skin permeation rate (47.14 μg/cm2/h) consisted of 0.5% piroxicam, 10% oleic acid, 60% Labrasol/ethanol (1:5) and water.
Similar content being viewed by others
References
Babar, A., Solanki, U. D., Cutie, A. J., and Plakogiannis, F., Piroxicam release from dermatological bases:In vitro studies using cellulose membrane and hairless mouse skin.Drug Dev. Ind. Pharm., 16, 523–540 (1990).
Baroli, B., Lopez-Quintela, M. A., Delgado-Charro, M. B., Fadda, A. M., and Blanco-Mendez, J., Microemulsions for topical delivery of 8-methoxsalen.J. Control Rel., 69, 209–218 (2000).
Buyuktimkin, N., Buyuktimkin, S., and Rytting, J. H., Chemical means of transdermal drug permeation enhancement. In: Ghosh, T.K., Pfister, W.R., Yum, S.I. (Eds.), Transdermal and Topical Drug Delivery Systems, Interpharm Press, IL, pp. 357–475 (1997).
Curdy, C., Kalia, Y. N., Naik, A., and Guy, R. H., Piroxicam delivery into human stratum corneumin vivo: iontophoresis versus passive diffusion.J. Control Rel., 76, 73–79 (2001).
Delgado-Charro, M. B., Iglesias-Vilas, G., Blanco-Mendez, J., Lopez-Quintela, M. A., Marty, J.-P., and Guy, R. H., Delivery of a hydrophilic solute through the skin from novel microemulsion systems.Eur. J. Pharm. Biopharm., 43, 37–42 (1997).
Doliwa, A., Santoyo, S., and Ygartua, P., Effect of passive and iontophoretic skin pretreatments with terpenes on thein vitro skin transport of piroxicam.Int. J. Pharm., 229, 37–44 (2001).
Gay, C. L., Green, P. G., Guy, R. H., and Francoeur, M. L., Iontophoretic delivery of piroxicam across the skinin vitro. J. Control Rel., 22, 57–67 (1992).
Gao, S. and Singh, J., Effect of oleic acid/ethanol and oleic acid/ propylene glycol on thein vitro percutaneous absorption of 5-fluorouracil and tamoxifen and the macroscopic barrier property of porcine epidermis.Int. J. Pharm., 165, 45–55 (1998).
Hsu, L. R., Huang, Y. B., Wu, P. C., and Tsai, Y. H., Percutaneous absorption of piroxicam from FAPG base through rat skin: effects of oleic acid and saturated fatty acid added to FAPG base.Drug Dev. Ind. Pharm., 20, 1425–1437 (1994).
Huang, Y.-B., Wu, P.-C., Ko, H.-M., and Tsai, Y.-H., Cardamom oil as a skin permeation enhancer for indomethacin, piroxicam and diclofenac.Int. J. Pharm., 126, 111–117 (1995).
Kanikkannan, N., Kandimalla, K., Lamba, S. S., and Singh, M., Structure-activity relationship of chemical penetration enhancers in transdermal drug delivery.Curr. Med. Chem., 7, 593–608 (2000).
Kawakami, K., Yoshikawa, T., Moroto, Y., Kanaoka, E., Takahashi, K., Nishihara, Y., and Masuda, K., Microemulsion formulation for enhanced absorption of poorly soluble drugs: I. Prescription design.J. Control Rel., 81, 65–74 (2002).
Kreilgaard, M., Influence of microemulsions on cutaneous drug delivery.Adv. Drug Deliv. Rev., 54, S77-S98 (2002).
Okuyama, H., Ikeda, Y., Kasai, S., Imamori, K., Takayama, K., and Nagai, T., Influence of non-ionic surfactants, pH and propylene glycol on percutaneous absorption of piroxicam from cataplasm.Int. J. Pharm., 186, 141–148 (1999).
Peltora, S., Saarinen-Savolanien, P., Kiesvaara, J., Suhonen, T.M. and Urtti, A., Microemulsion for topical delivery of estradiol.Int. J. Pharm., 254, 99–107 (2003).
Rhee, Y.-S., Choi, J.-G., Park, E.-S., and Chi, S.-C., Transdermal delivery of ketpoprofen using microemulsions.Int. J. Pharm., 228, 161–170 (2002).
Santoyo, S., Arellano, A., Ygartua, P., and Martin, C., Penetration enhancer effects on thein vitro percutaneous absorption of piroxicam through rat skin.Int. J. Pharm., 117, 219–224 (1995).
Santoyo, S. and Ygartua, P., Effect of skin pretreatment with fatty acids on percutaneous absorption and skin retention of piroxicam after its topical application.Eur. J. Pharm. Biopharm., 50, 245–250 (2000).
Schiantarelli, P. and Cadel, S., Piroxicam pharmacology activity and gastrointestinal damage by oral and rectal route.Arzneim-Forsch., 31, 87–92 (1981).
Schiantarelli, P., Cadel, S., Acerbi, D., and Pavesi, L., Anti-inflammatory activity and bioavailability of percutaneous piroxicam.Arzneim.-Forsch., 32, 230–235 (1982).
Tessari, L., Ceciliani, L., Belluati, A., Letizia, G., Martorana, U., Pagliara, L., Pognani, A., Thovez, G., Siclari, A., and Torri, G., Aceclofenac cream versus piroxicam cream in the treatment of patients with minor traumas and phlogistic affections of soft tissues: a double-blind study.Curr. Ther. Res., 56, 702–712 (1995).
Thacharodi, D. and Rao, K. P., Transdermal absorption of nifedipine from microemulsions of lipophilic skin penetration enhancers.Int. J. Pharm., 111, 235–240 (1994).
Troconiz, J. I., Lopez-Bustamante, L. G., and Fos, D., High-performance liquid chromatographic analysis of piroxicam and tenoxicam in plasma, blood and buffer solution. Application to pharmacokinetic studies in small laboratory animals.Arzneim-Forsch., 43, 679–681 (1993).
Tsai, Y.-H., Hsu, L.-R., and Naito, S.-I., Percutaneous absorption of piroxicam from ointment bases in rabbits.Int. J. Pharm., 24, 61–78 (1985).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, ES., Cui, Y., Yun, BJ. et al. Transdermal delivery of piroxicam using microemulsions. Arch Pharm Res 28, 243–248 (2005). https://doi.org/10.1007/BF02977723
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02977723