Abstract
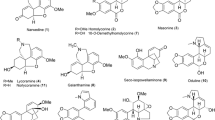
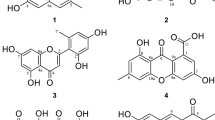
Activity-guided fractionation of a hexane-soluble extract of the roots ofLithospermum erythrorhizon, using a mouse brain monoamine oxidase (MAO) inhibition assay, led to the isolation of two known naphthoquinones, acetylshikonin and shikonin, and a furylhydroquinone, shikonofuran E. These compounds were shown to inhibit MAO with IC50 values of 10.0, 13.3, and 59.1 μM, respectively. Although no specificity for MAO-A and MAO-B was shown by acetylshikonin and shikonin, a Lineweaver-Burk plot analysis indicated that the inhibition was competitive for both MAO-A and MAO-B activity.
Similar content being viewed by others
References
Abell, C. W. and Kwan, S. W., Molecular characterization of monoamine oxidase A and B.Prog. Nucl. Acids Res. Mol. Biol., 65, 129–156 (2001).
Bach, A. W., Lan, N. C., Johnson, D. L., Abell, C. W., Bembenek, M. E., Kwan, S. W., Seeburg, P. H. and Shih, J. C., cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties.Proc. Natl. Acad. Sci. U.S.A., 85, 4934–4938 (1988).
Cho, M. H., Paik, Y. S., and Hahn, T. R. Physical stability of shikonin derivatives from the roots ofLithospermum erythrorhizon cultivated in Korea.J. Agric. Food Chem., 47, 4117–4120 (1999).
Foley, P., Gerlach, M., Youdim, M. B. H., and Riederer, P., MAO-B inhibitors: multiple roles in the therapy of neurodegenerative disorders?Parkinson Rel. Disorders, 6, 25–47 (2000).
Han, Y. N., Ryu, S. Y., and Han, B. H., Antioxidant activity of resveratrol closely relates with its monoamine oxidase-A inhibitory activity.Arch. Pharm. Res., 13, 132–135 (1990).
Inoue, K., Akaji, M., and Inouye, H. Quinones and related compounds in higher plants. XXI.: new findings on the proton and carbon-13 nuclear magnetic resonance spectra of shikonin.Chem. Pharm. Bull., 33, 3993–3997 (1985).
Jo, Y. S., Huong, D. T., Bae, K., Lee, M. K., and Kim, Y. H., Monoamine oxidase inhibitory coumarin fromZanthoxylum schinifolium.Planta Med., 68, 84–85 (2002).
Johnston, J. P., Some observations upon a new inhibitor of monoamine oxidases in brain tissue.Biochem. Pharmacol., 17, 1285–1297 (1968).
Knoll, J. and Magyar, K., Some puzzling pharmacological effects of monoamine oxidase inhibitors.Ad. Biochem. Psychopharmacol., 5, 393–417 (1972).
Kraml, M., A rapid microfluorimetric determination of monoamine oxidase.Biochem. Pharmacol., 14, 1684–1686 (1965).
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J., Protein measurement with the Folin phenol reagent.J. Biol. Chem., 193, 265–275 (1951).
Murphy, D. L., Substrate-selective monoamine oxidases.Biochem.Pharmacol., 27, 1889–1893 (1978).
Murphy, D. L., Sunderland, T., and Cohen, R. M., Monoamine oxidase-inhibiting antidepressants: A clinical update.Psychiatr. Clin. North Am., 7, 549–562 (1984).
Naoi, M. and Nagatsu, T., Quinoline and quininaldines as naturally occurring inhibitors specific for type A monoamine oxidase.Life Sci., 40, 1075–1082 (1987).
Nunez, M. B., Maguna, F. P., Okulik, N. B., and Castro, E. A., QSAR modeling of the MAO inhibitory activity of xanthones derivatives.Bioorg. Med. Chem. Lett., 14, 5611–5617 (2004).
Ro, J. S., Lee, S. S., Lee, K. S., and Lee, M. K., Inhibition of type A monoamine oxidase by coptisine in mouse brain.Life Sci., 70, 639–645 (2001).
Sankawa, U., Ebizuka, Y., Miyazaki, T., Isomura, Y. and Otsuka, H., Antitumor activity of shikonin and its derivatives.Chem. Pharm. Bull., 25, 2392–2395 (1977).
Suzuki, O., Katsumata, Y., Oya, M., Chart, V. M., Vermes, B., Wagner, H., and Hostettmann, K., Inhibition of type A and type B monoamine oxidases by naturally occurring xanthones.Planta Med., 42, 17–21 (1981).
Tanaka, S., Tajima, M., Tsukada, M., and Tabata, M., A comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkannin.J. Nat. Prod., 49, 466–469 (1986).
Tang, W. and Eisenbrand, G., Chinese drugs of plant origin. Springer-Verlag, New York, pp. 613–619 (1992).
Thull, U., Kneubuhler, S., Gaillard, P., Carrupt, P. A., Testa, B., Altomare, C., Carotti, A., Jenner, P., and McNaught, K. S., Inhibition of monoamine oxidase by isoquinoline derivatives: Qualitative and 3D-quantitative structure-activity relationships.Biochem. Pharmacol., 50, 869–877 (1995).
Wouters, J., Structural aspects of monoamine oxidase and its reversible inhibition.Curr. Med. Chem., 5, 137–162 (1998).
Yazaki, K., Fukui, H., and Tabata, M. Isolation of the intermediates and related metabolites of shikonin biosynthesis fromLithospermum erythrorhizon cell cultures.Chem. Pharm. Bull., 34, 2290–2293 (1986).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, W.H., Hong, S.S., Lee, S.A. et al. Monoamine oxidase inhibitory naphthoquinones from the roots ofLithospermum erythrorhizon . Arch Pharm Res 28, 400–404 (2005). https://doi.org/10.1007/BF02977668
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02977668