Abstract
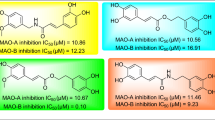
Four coumarins were isolated from chloroform extract of the root ofPeucedanum japonicum and identified as praeruptorin A (1), xanthotoxin (2), psoralen (3) and bergapten (4) on the basis of spectroscopic methods. The inhibitory activities of these coumarins on monoamine oxidase prepared by mouse brain were tested. The IC50 values of them were shown to be 27.4 μM (1), 40.7 μM (2), 35.8 μM (3), and 13.8 μM (4),in vitro.
Similar content being viewed by others
References Cited
Chen, I.-S., Chang, C.-T., Sheen, W.-S., Teng, C.-M., Tsai, I.-L., Duh, C.-Y. and Ko, F.-N., Coumarins and antiplatelet aggregation constituents from formosanPeucedanum japonicum.Phytochemistry 41, 525–530 (1996).
Choi, H. C., Rho, T. C., Kim, B. Y., Ko, H. R., Seung, C. K., Ahn, J. S. and Lee, H. S., Inhibition of nitric oxide production by coumarins fromPeucedanum japonicum in LPS-activated RAW 264.7 cells.Kor. J. Pharmacogn. submitted (1999).
Duh, C.-Y., Wang, S.-K. and Wu, Y.-C., Cytotoxic pyranocoumarins from aerial parts ofPeucedanum japonicum.Phytochemistry, 30, 2812–1814 (1991).
Duh, C.-Y., Wang, S.-K. and Wu, Y.-C., Cytotoxic pyranocoumarins from the roots ofPeucedanum japonicum.Phytochemistry, 31, 1829–1830 (1992).
Elgamal, M. H. A., Elewa, N. H., Elkhrisy, E. A. M. and Duddeck, H.,13C NMR chemical shifts and carbon-proton coupling constants of some furocoumarins and furochromones.Phytochemistry 18, 139–143 (1979).
Hossain, C. F., Okuyama, E. and Yamazaki, M., A new series of coumarin derivatives having monoamine oxidase inhibitory activity fromMonascus anka.Chem. Pharm. Bull., 44, 1535–1539 (1996).
Ikeshiro, Y., Mase, I. and Yomita, Y., Coumarin glycosides fromPeucedanum japonicum.Phytochemistry, 35, 1339–1341 (1994).
Kraml, M., A rapid microfluorometric determination of monoamine oxidase.Biochem. Pharmacol., 14, 1683–1685, 1965.
Masuda, T., Tagasugi, M. and Anetai, M., Psoralen and other linear furanocoumarins as phytoalexins in Glehnia littoralis.Phytochemistry, 47, 13–16, 1998.
Naoi, M., Takahashi, T., Darrez, H., Kabeya, R. and Taguchi, E., N-Methyl isoquinolinim ion as an inhibitor of tyrosine hydroxylase, aromatic L-amino acid decarboxylase and monoamine oxidase.Neurochem Int., 15, 315–320, 1989.
Okuyama, T. and Shibata, S., Studies on coumarins of a chinese drug “Gian-Hu”.Planta Med., 42, 89–96, 1981.
Perry, L. M.,Medicinal plants of east and southeast Asia. The MIT Press, Massachusetts, 1980.
Rendenbach-Muller, B., Schlecker, R., Traut, R. and Weifenbach, H., Synthesis of coumarins as subtype-selective inhibitors of monoamine oxidase.Bioorg. Med. Chem., 4, 1195–1198 (1994).
Rocha, L., Marston, A., Kaplan, M. A. C., Stoeckli-Evans, H., Thull, U., Testa, B. and Hosttmann, K., An antifungal γ-pyrone and xanthones with monoamine oxidase inhibitory activity from Hypericum brasiliense.Phytochemistry, 36, 1381–1385 (1994).
Rosazza, J. P. N., Duffel, M. W., El-Marakby, S., and Ahn, S. H., Metabolism of the Catharanthus griseus to monoamine oxidase B.J. Nat. Prod., 55, 269–284 (1992).
Schaufelberger, D. and Hostettmann, K., Chemistry and pharmacology ofGentiana lactea.Planta Med., 49, 219–221 (1988).
Yoshida, E., Fujimoto, H. and Yamazaki, M., Isolation of three new azaphilones, luteusins C, D, and E, from an Ascomycete,Talaromyces luteus.Chem. Pharm. Bull., 44, 284–287 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huong, D.T.L., Choi, H.C., Rho, T.C. et al. Inhibitory activity of monoamine oxidase by coumarins fromPeucedanum japonicum . Arch Pharm Res 22, 324–326 (1999). https://doi.org/10.1007/BF02976373
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02976373