Abstract
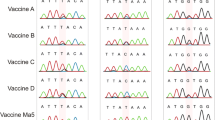
Oka strain VR-795 (Varicella Zoster Virus, VZV) of American Type Culture Collection (ATCC) has been used for chickenpox vaccine production. In order to use this strain for vaccine production, the strain must be identified and its stability must be confirmed. The identification of the Oka strain has been confirmed using Restriction Fragment Length Polymorphism (RFLP) and DNA sequence analysis of glycoprotein-II (gp-II). The amino acid sequences of Oka deduced from the DNA sequence of gp-II have changed at three amino acids against Ellen and at one amino acid against Webster. To prove the stability of the Oka strain during the passage, RFLP and DNA sequence analyses were also used with 11, 15 and 23 times of virus passage. We found that the Oka strain was stable at passages of up to 23 times, based on the RFLP and DNA sequence analyses. The confirmed Oka strain was renamed as BR-Oka for the purposes of chickenpox vaccine production.
Similar content being viewed by others
References
Davison, A. J. and Scott, J. E., The complete DNA sequence of varicella-zoster virus.J. Gen. Virol., 67, 1759–1816 (1986).
Hardy, I. and Gershon, A. A., Prospects for use of a varicella vaccine in adults.Infect. Dis. Clin. North Am., 4, 160–173 (1990).
Hayakawa, Y., Torigoe, S., Shiraki, K., Yamanishi, K. and Takahashi, M., Biological and biophysical markers of a live varicella vaccine strain (Oka): Identification of clinical isolates from vaccine recipients.J. Infect. Dis., 149, 956–963 (1984).
Hayakawa, Y., Yamamoto, T., Yamanishi, K. and Takahashi, M., Analysis of varicella-zoster virus DNAs of clinical isolates by endonuclease Hpal.J. Gen. Virol., 67, 1817–1829 (1986).
LaRussa, P., Lungo, O., Hardy, I., Gerrison, A. A., Steinberg, S. P. and Silverstein, S., Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates.J. Virol., 66, 1016–1020 (1992).
Martin, J. H., Dohner, D. E., Wellinghof, W. J. and Gelb, L. D., Restriction endonuclease analysis of varicellazoster vaccine virus and wild-type DNAs.J. Med. Virol., 9, 69–76 (1982).
Sambrook, J., Fritisch, E. F. and Maniatis, T.,Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1989.
Shiraki, K., Horiuchi, K., Asano, Y., Yamanishi, K. and Takahashi, M., Differentiation of Oka varicella vaccine strain from wild varicella-zoster virus strains isolated from vaccinees and household contact.J. Med. Virol., 33, 128–132 (1991).
Straus, S. E., Owens, J., Ruyechan, W. T., Takiff, H. E., Casey, T. A., Vande Woude, G. F., and Hay, J., Molecular cloning and physical mapping of varicella-zoster virus DNA.Proc. Natl. Acad. Sci. USA., 79, 993–997 (1982).
Takahashi, M., Otsuka, T., Okuno, Y., Asano, Y., Yazaki, T. and Isomura, S., Live vaccine used to prevent the spread of varicella in children in hospital.Lancet 2, 1288–1290 (1974).
Takahashi, M., Okuno, Y., Ostsuka, T., Osame, J., Takamizawa, A., Sasada, T. and Kubo, T., Development of a live attenuated varicella vaccine.Biken J. 18, 25–33 (1975).
Takahashi, M.,Advances in virus research, American Press, Vol 26, 285p-355p (1983).
Yoshizo, A., Takao, N., Takao, M., Takehiko, Y., Shigemitsu, I., Koichi, Y. and Michiaki, T., Long term protective immunity of recipients of the Oka strain of live varicella vaccine.PEDIATRICS, 75(4), 667–671 (1985).
Weller, T. H., Serial propagation in vitro of agents producing inclusion bodies derived from varicella and herpes zoster.Proc. Soc. Exp. Bio. Med. 83, 340–346 (1953).
White, C., Barbara, J., K., Carol, S., H., Kathryn, L., I., Varicella vaccine, VARIVAX, in health children and adolescents: Result from clinical trials, 1987 to 1989.PEDIATRICS 87 (5), 604–610 (1991)
Wolfgang, K. J.,Virology, 3rd ED., Prentice-Hall, Englewood Cliffs, New Jersey, (1988).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, SM., Song, SW., Kim, SL. et al. Comparison between of the attenuated BR-Oka and the wild type strain of Varicella Zoster Virus (VZV) on the DNA level. Arch Pharm Res 23, 418–423 (2000). https://doi.org/10.1007/BF02975458
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02975458