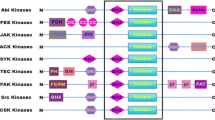
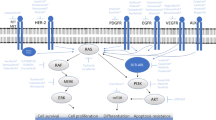
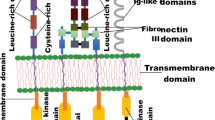
Abstract
Attempts to develop drugs, specific for cancer cells, are dealt here according to the intended cell-target. While many target specific drugs were developed, they reach only moderate successes in clinics for reasons, such as, delivery problem, lack of in vivo efficacy or toxicity. However, recent efforts focusing on the diversity of tyrosine kinases, participating in cell-signal transduction, brought fruit. The firs such drug, Givec, approved by the USFDA recently, is used in clinics with great success to threat CML The drug inhibits tyrosin kinase of bcr-abl, c-abl and v-abl. Work is progressing on other tyrosin kinase inhibitors and on other type of specific cancer cell signal protein inhibitors. These efforts are hoped to yield better cures for cancer in the near future.
Similar content being viewed by others
References
Actis, A. M., Caruso, S. P., and Levin, E., Opposite effect of a cAMP analogue on tumor growth related to hormone dependence of a murine mammary tumor. Cancer Lett., 96 81–85 (1995).
Antoniades, H. N., Galanopoulos, T., Neville-Golden, J., and OHara CJ., Malignant epithelal cells in primary human lung carcinomas coexpressed in vivo platlet-derived growth factor (PDGF) and PDGF receptor mRNA and their protein products. Proc. Natl. Acad. Sci., 89, 3942–3946 (1992).
Asiedu, C., Biggs, J., Lilly, M., and Kraft, A. S., Inhibition of leukemic cell growth by the protein kinase C activator bryostatin 1 corre-lates with the dephosphorylation of cyclin-dependent kinase 2. Cancer Res., 55, 3716–3720 (1995).
Bacus, S. S., Stancovski, I., Huberman, E., Chin, D., Hurwitz, E., Mills, G. B., Ullrich, A., Sela, M., and Yarden, Y., Tumorinhibitory monoclonal antibodies to the HER-2/Neu receptor induce differentiation of human breast cancer cells. Cancer Res., 52, 2580–2589 (1992).
Beran, M., Caox, X., Csitrov, Z., Jeha, S., Jin, G., OBrien, S., Talpaz, M., Arlinghause, R. B., Lydon, N. B., and Kartarjian, H., Selective inhibition of cell proliferation and BCR-ABL phosphorylation in acute lymphoblastic leukemia cells expressing M: 190,00 BCR-ABL proteins by a tyrosine kinase inhibitor (CGP 57148). Clinical Cancer Res., 4, 1661–1672 (1998).
Bishop, J. M., The molecular genetics of cancer. Science, 235, 305–311 (1987).
Bishop, W. R., Patton, R., Bohanon, S., Bergman, G., Meyers, M., Braun, C., Khuri, F., and Kirschmeier, P., Evaluation of bio-chemical markers of protein farnesylation in human tumor speci-mens following treatment with oral SCH66336, an inhibitor of famesyl protein transferases. Proc. Am. Assoc. Cancer Res., 42, 259 (2001).
Blau, C. A., Constantoulakis, P., Shaw, C. M., and Stamatoyannopoulos, G., Fetal hemoglobin induction with butyric acid: efficacy and toxicity. Blood, 81, 529–37 (1993).
Bleckburn, E. H., Telomerases. Ann. Rev. Biochem., 61, 113–129 (1992).
Boe, R., Grejtsen, B. T., Doskeland, S. O., and Vintermyr, O. K., 8-CI-cAMP induces apoptotic cell death in a mam-mary carcinoma cell (MCF-7) line. Br. J. Cancer, 72, 1151–1159 (1995).
Buser, C. A., Anthony, N., Bell, I. Dinsmore, C., Gibba, J., Graham, S., Hartman, S., Kohl, N., Lobbel, R., Lumma, B., Williams, T., and Huber, H., LC/MS Characterization of Ki-Ras prenylation after treatment with selective and dual inhibitors of FPTase and GGPT ase-l in pre-clinical mouse model. Proc. Am. Assoc. Cancer Res., 42, 486 (2001).
Budillon, A., Guarrasi, R., Gennaro, E. D., Bruzzese, F., Errico, S., Pirozzi, G., Caraglia, M., Avallone, A., Tassonel, P. F., Lombardi, M. L., Caponigro, F., Venuta., and Tagia-ferri, P., ZD1839 (“Iressa”), an EGFR tyrosine kinase inhi-bitor, potentiates non-Mhc restricted cytotoxicity in human cancer cell lines. Proc. Am. Assoc. Cancer Res., 42, 802 (2001).
Buguet-Fagot, C., Lallemand, F., Charollais, R.H., and Mester, J., Sodium butyrate inhibits the phosphorylation of the retinobla-stoma gene product in mouse fibroblasts by a transcription dependent mechanism. J. Cell. Physiol., 166, 631–636 (1966).
Carducci, M., Bowling, M., Eisenberger, M., Sinibaldi, V., Simons, S., Chen, T., Noc, D., Graham, L., and Done-hover, R., Phenyl-butyrate (PB) for refractory solid tumors: A phase I clinical and pharmacological evaluation. Proc. Am. Assoc. Cancer Res., 37, 371–376 (1996).
Caroll, M., Ohno-Jones, S., Tamura, S., Buchdunger, E., Zimmermann, J., Lydon, N. B., and Gilliland, D. G., CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL and TEL-PDGFR fusion proteins. Blood, 90, 4947–4952 (1997).
Ciardiello, F., Caputo, R., Bianco, R., Damiano, V., Pomatico, G., De Placido, S., Bianco, A. R., and Tortora, G., Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selec-tive tyrosine kinase inhibitor. Clin. Cancer Res., 6, 2053–2059 (2000).
Cobb, M. H., and Goldsmith, E. J., How MAP kinases are regulated. J. Biol. Chem. 270, 14843–14846 (1995).
De Luca, L. M., Retinoids and their receptors in differentiation, embriogenesis and neoplasia. FASEB J., 5, 2924–2933 (1991).
Deninger, M. W., Goldman, J. M. V., Lydon, N., and Melo, J. V., The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR-ABL-positive cells. Blood, 90, 3691–3698 (1997).
Derexler, H. G., Gignak, S. M., Jones, R. A., Scott, C. S., Pettit, G. R., and Hoffbrand, A. V., Bryostatin 1 induces differentiation of B-chronic lymphocytic leukemia cells. Blood, 74, 1747–1757 (1989).
de The, H., Vivanco-Ruiz, M., Tiollais, P., Stunnenberg, H., and Dejean, A., Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature, 343, 177–180 (1990).
Druker, B. J., Tamura, S., Buchdunger, E., Ohno, T., Segal, G. M., Fannings, S., Zimmermann, J., and Lydon, N. B., Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nature Medicine, 2, 561–568 (1996).
Ezzat, S., Kontogeorgos, G., Redelmeier, D. A., Horvath, E., Harvis, A. G., and Kovacs K., In vivo responsiveness of morpho-logical variants of growth hormone-producing pituitary adenoma to octreotide. Eur. J. Endoctrinol., 133, 686–690 (1995).
Fletcher, T. M. and Chen, S. F., The effect of 7-deaza-2-deoxyguanosine-5-triphosphate and 7-deaza-2deoxyadenosine-5-tri-phosphate on telomerase activity. Ann. Oncol., 7 (suppl 1), 71 (1996).
Franke, T. F., Kaplan, D. R., Cantley, LC., and Toker, A., Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-biphosphate. Science, 275, 665–668 (1997).
Fritz, J., Waechter, M., Zang, C., von Deimling, A., Possinger, K., Reichart, J., Koeffler, P., and Elstner, E., Combination of pioglita-zone (PGZ) and all-trans-retinoic acid (ATRA) inhibits clonal growth and induces apoptosis of cancer cells. Proc. Am. Assoc. Cancer Res., 42, 445 (2001).
Fujioka, T., Ishikura, K., Hasegawa, M., Ogyu, K., Matsushita, Y., Sato, M., Sato, F., Aoki, H., and Kubo, T., Antimumor effect of oral administration of an interferon-inducing pyrimidinone, bropirimine, on murine renal-cell carcinoma. Cancer Chemother. Pharmacol., 36, 7–12 (1995).
Hahn, W. C., Counter, C. M., Lundberg, A. S., Beijersbergen, R. L., Brooks, M. W., and Weinberg, R. A., Creation of human tumor cells with defined genetic elements. Nature, 400, 464–468 (1999).
Han, Z., Chatterjee, D., Early, J., Pantazis, P., Hendrickson, E. A., and Wyche, J.H., Isolation and characterization of an apoptosis resistant variant of human leukemia HL-60 cells that has switched expression from Bcl-2 to Bcl-xI. Cancer Res. 56, 1621–2628 (1996).
Harley, C. B., Kim, N. W., Prowse, K., Weinrich, S. L., Hirsch, K. S., West, M. D., Bacchetti, S., Hirte, H. W., Counter, C. M., and C. W., Telomerase, cell immortality and cancer. Cold Sping Harbor Symp. Quant. Biol., 59, 307–315 (1994).
Hiyama, E., Hiyama, R., Yokoiama, T., Matsuura, Y., Piatszek, M. A., and Shay, J. W., Correlating telo-merase activity levels with human neuroblastoma out-comes. Nature Med., 1, 249–255 (1995).
Hiyama, E., Yokoyama, T., Tatsumoto, N., Hiyamaka, K., Imamura, Y., Murakami, Y., Kadama, T., Piatszek, M. A., Shay, K., and Matsuura, Y., Telomerase activity in gastric cancer. Cancer Res., 55, 3258–3262 (1995).
Hohl, R. J., Lewis-Tisebar, K., Pogatchik, D. M., and Wiemer, D. F., Structural requirement for inhibition of farnesyl protein trans-ferase by isoprenoid phosphonic acid. Proc. Am. Assoc. Cancer Res. 37, 426 (1996).
Ibrahim, S., Peggins, J., Knapton, A., Licht, T., and Aszalos, A., Influence of antipsychotic, antiemetic, and Ca2+ channel blocker drugs on the cellular accumulation of the anticancer drug daunorubicine: P-glycoprotein modulation, J. Pharmaco. Exper, Therapeutics 295, 1276–183 (2000).
Kaur, G., Stetler-Stevenson, M., Sebers, S., Worland, P., Sedlacek, H., Myers, C., Czech, J., Naik, R., and Sausville, E., Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J. Natl. Cancer Inst., 84, 1736–1740 (1992).
Kim, E., Glisson, B. S., Meyers, M. L., Herbert, R. S., Shin, D. M., Statkevich, P., Bangert, S., Hong, W. K., and Khuri, F.R., A phase I/II study of the farnesyl transferase inhibitor (FTI) SCH66336 with paclitaxel in patients with solid tumors. Proc. Am. Assoc. Cancer Res., 42, 488 (2001).
Kim, N. W., Piatyszek, M. A., Prowse, K. R., Harley, C. B., West, M. D., Ho, P. L., Coviello, G. M., Wright, W.E., Weinrich, S. L., and Shay, J. W., Specific association of human telomerase activity with immortal cells and cancer. Science, 266, 2011–2015 (1994).
Kraemer, K. H., DiGiovanna, J. J., Moshell, A. N., Tarone, R. E., and Peck, G. L., Prevention of skin cancer in xerodema pigmentosum with the use of oral isoretinoin. N. Engl. J. Med., 318, 1633–1637 (1988).
Krupitza, G., Grill, S., Harant, H., Hulla, W., Szekeres, T., Huber, H., and Dittrich, C., Genes related to growth and invasivness are repressed by sodium butyrate in ovarian carcinom cells. Br. J. Cancer, 73, 433–438 (1966).
Lamberts, S. W. J., Koper, J. W., and Reubi, J. C., Potential role of somatostatin analogues in the treatment of cancer. J. Clin. Invest., 17, 281–287 (1987).
IeCountre, P., Mologni, L., Cleris, L., Marschesi, E., Buchdunger, E., Giardini, R., Formelli, F., and Gambacorti-Passerini, C., In vivo eradication of human BCR/ABL-positive leukemia cells with ABL kinase inhibitor. J. Natl. Cancer Inst., 91, 163–168 (1999).
Leftheris, K., Kline, T., Vite., G. D., Cho, Y. M., Bhide, R. S., Patel, D. V., Schmidt, P. J., Weller, H. N., Andahazy, M. L., Carboni, J. M., Gullo-Braun, J. L., Lee, F. Y., Ricca, C., Rose, W. C., Development of highly potent inhibitors of Ras famesyl-transferase possessing cellular and in vivo activity, J. Med. Chem., 39, 224–236 (1996)
Levitzki, A. and Gazit, A., Tyrosine kinase inhibition: An approach to drug development. Science, 267, 1782–1788 (1995).
Liebow, C., Reilly, C., Serrano, M., and Schally, A. V., Somatostatin analogs inhibit growth of pancreatic cancer by stimulating tyro-sine phosphatase. Proc. Natl. Acad. Sci., 86, 2003–2007 (1989).
Lippman, S. M., Clark, L. C., and Parkinson, D., Pharmacologic prevention and therapy of skin cancer. In: Chemo and immuno prevention of cancer. Pastorino, U. and Hong, W.K.: eds, George Thiem Verlag, Stuttgart, 1991, pp 177–187.
Lotzava, E., Savary, C. A., and Stringfellow, D.A., 5-halo-6- phenyl pyrimidinones: new molecules with cancer therapeutic potential and interferon-inducing capacity are strong inducer of murine natural killer cells. J. Immunol., 130, 965–969 (1983).
Mahon, F. V., Deninger, M. W. N., Schultheis, B. Chabrol, J., Reiffers, J., Geldman, J. M., and Melo, J. V., Selection and characterizatin of BCR-ABL positive cell lines with different selectivity to tyrosine kinase inhibitor imatinib: divers mechanisms of resistance. Blood, 96, 10701079 (2000).
Main, J. P., Chen, S. E., and Windle, B., Interaction between telo-merase oligonucleotide primers and CHO non-processive telo-merase. Proc. Am. Soc. Cancer Res., 36, 561 (1996).
Maki, A., Diwakaran, H., Redman, B., Al-Asfors, M., Pettit, G. R., Mohammed, R. M., and Al-Katib, A., The bcl-2 and p53 oncopro-teins can be modulated by bryostatin 1 and dolastatins in human diffuse large cell lymphoma. Anticancer Drugs, 6, 392–397 (1995).
Malik, S., Brattain, M., Rowinsky, E., Miller, A., Duffey, D., de Graffenried, L., Siu, L., Simmons, C., Kreisberg, J., Nadler, P., and Hidalgo, M., Inhibition of the epidermal growth factor receptor (EGFR) activation and signaling by OSI-774, a novel EGFR inhibitor, in clinical specimens of head and neck carcinoma. Proc. Am. Assoc. Cancer Res., 42, 852 (2001).
Mason, W., Malkin, M., Lieberman, F., Cropp, G., and Hannah, A., Pharmacokinetics of SU101, a novel signal transduction inhi-bitor, in patients with recurrent malignant glioma. Proc. Am. Assoc. Cancer Res., 37, 166–167 (1996).
Miller, A. A., Kurschel, E., Osieka, R., and Schmidt, C. G., Clinical pharmacology of sodium butyrate in patients with acute leukemia. Eur. J. Clin. Oncol., 23, 1283–1287 (1987)
Mohammed, R. M., Diwakaran, H., Maki, A., Emara, M. A., Pettit, G. R., Redman, B., and Al-Katib, A., Bryostatin induces apoptosis and augments inhibitory effects of vincristin in human diffuse large cells. Leuk. Res., 19, 667–673 (1995).
Moulder, S. L., Yakes, F. M., Bianco, R., and Arteaga, C. L., A rational for the use of small molecule EGF receptor tyrosine kinase inhibitors against HER2/neu (erbB-2) overexpressing breast tumor cells. Proc. Am. Assoc. Cancer Res., 42, 853 (2001).
Muindi, J., Frankel, S. R., Miller, W. H., Jakubowski, A., Scheinberg, D. A., Young, C. W., Dimitrovsky, E., and Warress, R. P. Jr., Continuous treatment with all-trans retinoic acid causes a progressive reduction for reduction in plasma drug concentration: Implication for relapse and retinoid resistance in patients with acute promyelocytic leukemia. Blood, 79, 299–303 (1992).
Mullin, R. J., Alligood, K. J., Allen, P. P., Crosby, R. F. M., Keith, B. R., Lakckey, K., Gillmer, T. M., Griffin, R. J., Murray, D. M., and Tadepalli, S. M., Antitumor activity of GW2016 in the EGFR positive human head and neck cancer xenograft, HN5. Proc. Am. Assoc. Cancer Res., 42, 854 (2001).
Napoli, J. L., The biogenesis of retinoic acid: a physiologically significant promoter of differentiation. In: Chemistry and biology of retinoic acid. Dawson, M.I. and Okamura, H.: eds, CRC Press, Boca Raton, Fl, 1990, pp 229–249.
North, P. S., Davies, S. L., Ciardiello, F., Damiano, V., Bianco, C., Pepe, S., Bianco, A. R., Harris, A. L., Hickson, I. D., and Tortora, G., Overexpression of the Rl alpha subunit of protein kinase A confers hypersensitivity to topoisomerase II inhibitors and 8-CI-cyclic adenosine 3,5- monophosphate in Chinese hamster ovary cells. Cancer Res., 54, 4123–4128 (1994).
Novogrodsky, A., Dvir, A., Ravid, A., Shkolnik, T., Stemel, K. H., Rubin, A. L., and Zaizov, R., Effect of polar organic compounds on myeloid cells: butyrate induced partial remission of acute myelogenous leukemia in a child. Cancer, 51, 9–14 (1983).
Nudelman, A., Ruse, A., Aviram, A., Rabizadeh, E., Shaklai, M., Zimrah, Y., and Raphaeli, A., Novel anticancer prodrug of butyric acid. J. Med. Chem. 35, 687–694 (1992).
Patnaic, A., Izbicka, E., Eckhardt, S. G., Davidson, K., Goetz, A., McCreeny, H., Tolcher, A., Mori, M., Terada, K., Bal, K., Rybak, A., Thibault, H., Richards, L., Gentler, L., and Rowinsky, E., Inhibition of HDJ2 protein farnesylation in peripheral blood mononuclear cells as a pharmacodynamic endpoint in a phase I study of R115777 and gemcitabine. Proc. Am. Assoc. Cancer Res., 42, 488 (2001).
Perkins, A. S. and Stern, D. F., Molecular Biology of Cancer: Oncogens. In Cancer: Principle and Practice of Oncology, De Vita, V. T., Hellman, S. and Rosenberg, S. A.: eds. Lippincott, Philadelphia, 1997, pp 79–102.
Pettit, G. R., The bryostatins. In progress in the chemistry of organic natural products. Herz, W., Kirby, G.W., Steglich, W. and Tamm, C.H.: eds, Spinger, Vienna, 1991, pp 153–195.
Philip, P. A., Rea, D., Thavasu, P., Crmichael, J., Stuart, N.S., Rokett, H., Talbot, D.C., Ganesau, T., Pettit, G. R., and Balkwill, F., Phase I study of bryostatin 1: Assessment of interlukin 6 and tumor necrosis factor alpha induction in vivo. J. Natl. Cancer Inst., 85, 1812–1818 (1993).
Ramage, A. D., Langdon, S. P., Ritchie, A. A., Burns, D. J., and Miller, W. R., Growth inhibition by 8-Cloro-cyclic AMP of human HT29 colorectal and ZR-75-1 breast carcinoma xenografts is associated with selective modulation of protein kinase A isoenzymes. Europ. J. Cancer, 31A, 969–973 (1995).
Rambaldi, A., Biondi, A., Pandolfi, P. P., Torcia, M., Bettoni, S., Vannier, E., Barbui, T., Shaw, A. B., Dinerello, C. A., and Cozzolino, F., Modulation of cell proliferation and cytokine production in acute myeloblastic leukemia by interlukin-1 receptor antagonist and lack of its expression. Blood, 78, 3248–3253 (1991).
Raphaeli, A., Rabizadeh, E., Aviram, A., Shaklai, M., Ruse, M., and Nudelman, A., Derivatives of butyric acid as potential antineo-plastic agents. Int. J. Cancer, 49, 66–72 (1991).
Riou, J. F., Mailliet, P., Laoui, A., Renou, E., Petitgenet, O., Guittat, L., and Mergny, J., Apoptosis, cell senescence and telomerase shortening induced by a new series of specific G- quadruplex DNA ligand. Proc. Am. Assoc. Cancer Res., 42, 837 (2001)
Rosato, R. R., Almenara, J. A., Cartee, L. A., and Grant, S., The cydine-dependent kinase inhibitor flavopiridol disrupts sodium butyrate-induced p21waf-1CIP1 expression and enhances apopto-sis in human myeloid leukemia cells. Proc. Am. Assoc. Cancer Res., 42, 437 (2001).
Santini, V., Lamberts, S. W., Krenning, E. P., Bachx, B., and Lowenberg, E., Somatostatin and its cyclic octapeptide analo-gue SMS 201-995 as inhibitor of proliferation of human acute lymphoblastic and acute myeloid leukemia. Leuk. Res., 19, 707–712 (1995).
Sarosdy, M. F., Lamm, D. L., Williams, R. D., Moon, T. D., Flanigen, R. C., Crawford, E. D., Wilks, N. E., Earhart, R. H., and Merritt, J. A., Phase I trial of oral bropirimine in superficial bladder cancer. J. Urology, 147, 31–33 (1992).
Sawyers, C., Hochhaus, A., Feldman, E., Goldman, J. M., Miller, C., Ben-Am, M., Capdenlle, R., and Dumber, B., A phase II study to determine the safety and anileukemic effects of ST1571 in patients with Philadelphia chromosome positive chronic myeloid leukemia in myeloid blast crisis. Blood, 96, 503a (2000).
Sebold-Leopold, J. S., Govan, R. C., Gibbs, B. S., Przybranowski, S., Latash, M., Leopold, W. R., Scholten, J., Zimmerman, K., Hupe, D., Leonard, D., McNamara, D., Bur, S., Bolton, G. L., and Doherty, A. M., Biological evaluation of cell permeable inhibitors of ras famesyl trasferase. Proc. Am. Assoc. Cancer Res., 37, 423 (1996).
Shapiro, G. I. and Harper, J. W., Anticancer drug targets: cell cycle and checkpoint control J. Clin. Invest., 104, 1645–1653 (1999).
Smith, V., Brunton, L., Valenti, M., Johnson, S., Workman, P., and Kelland, L., Preclinical antitumor and pharmacodynamic studies with the famesyl transferase inhibitor (FTI) R115777. Proc. Am. Assoc. Cancer Res., 42, 260 (2001).
Steube, K. G. and Dexler, H. G., The protein kinase activator bryostatin 1 induces the rapid release of TNF alpha from MONO-MAC-6 cells. Biochem. Biophys. Res. Commun., 214, 1197–1203 (1995).
Stringfellow, D. A., Vanderberg, H. C., and Weed, S.D., Interferon induction by 5-halo-6-phenyl pyrimidinones. J. Interferon Res., 1, 1–14 (1980).
Sun, J., Ohkanda, J., Adnane, J., Lockman, J., Hamilton, A., and Sebti, S. M., Geranylgeranyltransferase I (GGTase I) inhibitors induce mammary tumor regression in oncogenic H- ras transgenic mice: validation of GGTase I as a cancer therapy target. Proc. Am. Assoc. Cancer Res., 42, 260 (2001).
Sun, J., Pei, Z., and Sebti, S. M., Stable expression of a 5’400 bp antisense of the beta subunit of farnesyltransferase in human lung carcinoma blocks oncogenic signaling in vitro and in vivo. Proc. Am. Assoc. Cancer Res., 37, 419 (1996).
Sun, J., Quian, Y., Hamilton, A. D., and Sebti, S. M., Ras CAAX peptidomimetic FTI-276 selectively blocks tumor growth in nude mice of a human lung carcinoma with K-ras mutation and p53 deletion. Cancer Res., 55, 4243–4247 (1995).
Tong, K. P., De Weese, T. L., Mansfield, E. P., and Carducci, M.A., New Insights into phenylbutyrate bioactivity in human prostate cancer. Proc. Am. Assoc. Cancer Res., 37, 361 (1996).
Vintermyr, O. K., Boe, R., Brustung, O. T., Maronde, E., Aakvaag, A., and Doskeland, S. O., Cyclic adenosine monophosphate (cAMP) analog 8-CI- and 8-NHZ-cAMP induce cell death independently of cAMP kinase-mediated inhibition of the G1/S transition in mammary carcinoma cells (MCF-7). Endocrinology, 136, 1523–2520 (1995).
Wierenga, W., Antiviral and other bioactivities of pyrimidinones. Pharm. Ther., 30, 67–89 (1985).
Yoneda, T., Lyall, R. M., Alsina, M. M., Persons, P. E., Spada, A. P., Levizki, A., Zilberstein, A., and Mundy, G. M., The antiproliferative effects of tyrosine kinase inhibitors tyrphostins on a human squamous carcinoma in vitro and in nude mice. Cancer Res., 51, 4430–4435 (1991).
Yoshida, H. N., Kuniyasu, H., Yasui, W., Kitadai, Y., Toge, T., and Tahara, E., Expression of growth factors and their receptors in human esophageal carcinomas: regulation of expression by epidermal growth factor and transforming growth factor alpha. J. Cancer Res. Clin. Oncol., 19, 401–407 (1003).
Zhang, L., Gary, E., Gallik, G. E., and Donato, N. J., Apoptotic sen-sitization in non-small cell lung cancer cells through tyrosine kinase inhibition: possible role for cytokine signaling and non-receptor tyrosine kinase as regulators of caspase activation and NSCLC cell survival. Proc. Am. Assoc. Cancer Res., 42, 131 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aszalos, A. Specific cell-signal targets for cancer chemotherapy. Arch Pharm Res 25, 1–10 (2002). https://doi.org/10.1007/BF02975254
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02975254