Abstract
Background
A prospective randomized multi-center study was undertaken for 2 years and 3 months from November 1982, with the aim of examining the significance of using a combination of futraful (FT) and tamoxifen (TAM) for postoperative adjuvant therapy for stage II breast cancer after curative surgery.
Methods
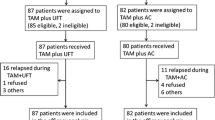
Patients were divided into two groups and received one of the following treatment protocols: treatment A, intravenous administration of doxorubicin (DOX) 20 mg/body on the day of surgery and 10 mg/body the next day, followed by oral FT 600 mg/day for 2 years from the 14th day after surgery; treatment B, the same pattern of DOX administration followed by combined therapy with FT and TAM 20 mg/day for 2 years. The number of patients was 428 (treatment A 210 and treatment B 218), of whom 418 (97.7%) were followed for 10 years for analysis.
Results
Significantly higher 5- and 10-year overall survival (OS) rates were observed with treatment B compared with treatment A (p = 0.0101 and 0.0219). Node-positive patients appeared to derive more benefit from TAM than node-negative patients. The difference in 10-year OS between treatment A and treatment B was more evident than that of the 5-year OS in patients with more than 4 positive nodes (p = 0.0313 vs. 0.0479). No increase in adverse reactions was seen as a result of combining TAM with FT.
Conclusion
The study results demonstrate that for stage II breast cancer concomitant administration of FT and TAM is superior to FT alone for postoperative adjuvant therapy, and administration of TAM for 2 years may contribute not only to 5-year survival rates but also to 10-year survival rates of node-positive patients.
Similar content being viewed by others
Abbreviations
- FT:
-
Futraful
- TAM:
-
Tamoxifen
- DOX:
-
Doxorubicin
- 5-FU:
-
5-Fluorouracil
- ER:
-
Estrogen receptor
- DCC:
-
Dextran-coated charcoal
- OS:
-
Overall survival
- RFS:
-
Relapse-free survival
References
Early Breast Cancer Trialists’ Collaborative Group: Tamoxifen for early breast cancer: an overview of the randomised trials.Lancet 351: 1451–1467, 1998.
Bianco AF, De Placido S, Gallo C,et al: Adjuvant therapy with tamoxifen in operable breast cancer. 10 year results of Naples (GUN) study.Lancet 2: 1095–1099, 1988.
Abe O: The role of chemoendocrine agents in postoperative adjuvant therapy for breast cancer: metaanalysis of the first collaborative studies of postoperative adjuvant chemoendocrine therapy for breast cancer (ACETBC).Breast Cancer 1: 1–9, 1994.
Uchino J, Samejima N, Tanabe T,et al: Positive effect of tamoxifen as part of adjuvant chemo-endocrine therapy for breast cancer.Br J Cancer 69: 767–771, 1994.
Carter SK: Chemotherapy of breast cancer; Current status. In: Meuson JC ed, Breast Cancer Trends in Research and Treatment, Raven Press, New York, pp 193–215, 1976.
Andersson M, Kamy C, Jensen M-B,et al: Tamoxifen in high-risk premenopausal women with primary breast cancer receiving adjuvant chemotherapy. Report from the Danish Breast Cancer Co-operative Group DBCG 82B trial.Eur J Cancer 35: 1659–1666, 1999.
Yoshida M, Abe O, Uchino J,et al: Meta-analysis of second collaborative study of adjuvant chemoendocrine therapy for breast cancer (ACETBC) in patients with stage II, estrogen-receptor-positive breast cancer.Breast Cancer 4: 93–101, 1997.
Aapro MS: Adjuvant therapy of primary breast cancer: a review of key findings from the 7th International Conference, St. Gallen, February 2001.Oncologist 6: 376–385, 2001.
Vorgias G, Koukouras D, Tzoracoeleftherkis E,et al: Adjuvant tamoxifen versus tamoxifen plus CMF in the treatment of early breast cancer in Greece. Fifteenyear results of a randomised prospective trial and the potential risks of the antioestrogen.Anticancer Res 20: 3849–3854, 2000.
Author information
Authors and Affiliations
Additional information
Reprint requests to Yoshinobu Hata, Sapporo Social Insurance General Hospital, 2-6 Atsubefsu-Chuo, Atsubetsu-Ku, Sapporo 004-8618, Japan.
About this article
Cite this article
Hata, Y., Takahashi, H., Todo, S. et al. Ten-year results of a randomized trial on adjuvant chemo-endocrine therapy with tamoxifen for stage II breast cancer. Breast Cancer 10, 134–139 (2003). https://doi.org/10.1007/BF02967638
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02967638